Myeloid-Derived Suppressor Cell Accumulation Drives Intestinal Fibrosis through mCCL6/hCCL15 Chemokine-Mediated Fibroblast Activation
- PMID: 39739231
- PMCID: PMC11848553
- DOI: 10.1002/advs.202411711
Myeloid-Derived Suppressor Cell Accumulation Drives Intestinal Fibrosis through mCCL6/hCCL15 Chemokine-Mediated Fibroblast Activation
Abstract
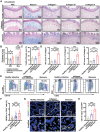
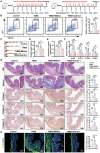
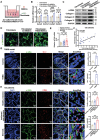
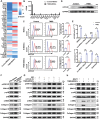
Intestinal fibrosis, a severe complication of Crohn's disease (CD), is linked to chronic inflammation, but the precise mechanism by which immune-driven intestinal inflammation leads to fibrosis development is not fully understood. This study investigates the role of myeloid-derived suppressor cells (MDSCs) in intestinal fibrosis in CD patients and a 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced mouse model. Elevated MDSCs are observed in inflamed intestinal tissues prior to fibrosis and their sustained presence in fibrotic tissues of both CD patients and murine models. Depletion of MDSCs significantly reduces fibrosis, highlighting their key role in the fibrotic process. Mechanistically, MDSC-derived mCCL6 activates fibroblasts via the CCR1-MAPK signaling, and interventions targeting this axis, including neutralizing antibodies, a CCR1 antagonist, or fibroblast-specific Ccr1 knockout mice reduce fibrosis. In CD patients with stenosis, human CCL15, analogous to mCCL6, is found to be elevated in MDSCs and activated fibroblasts. Additionally, CXCR2 and CCR2 ligands are identified as key mediators of MDSC recruitment in intestinal fibrosis. Blocking MDSC recruitment with CXCR2 and CCR2 antagonists alleviates intestinal fibrosis. These findings suggest that strategies targeting MDSC recruitment and mCCL6/hCCL15 signaling could offer therapeutic benefits for intestinal fibrosis.
Keywords: IBD; MDSCs; intestinal fibroblasts; intestinal fibrosis; mCCL6/hCCL15.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- D'Alessio S., Ungaro F., Noviello D., Lovisa S., Peyrin‐Biroulet L., Danese S., Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 169. - PubMed
-
- Schulberg J. D., Wright E. K., Holt B. A., Hamilton A. L., Sutherland T. R., Ross A. L., Vogrin S., Miller A. M., Connell W. C., Lust M., Ding N. S., Moore G. T., Bell S. J., Shelton E., Christensen B., De Cruz P., Rong Y. J., Kamm M. A., Lancet Gastroenterol. Hepatol. 2022, 7, 318. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
