Personalized multifactorial risk assessment in neoadjuvant-treated breast carcinoma
- PMID: 39739270
- PMCID: PMC11930868
- DOI: 10.1007/s10549-024-07584-4
Personalized multifactorial risk assessment in neoadjuvant-treated breast carcinoma
Abstract
Purpose: Due to biological heterogeneity of breast carcinoma, predicting the individual response to neoadjuvant treatment (NAT) is complex. Consequently, there are no comprehensive, generally accepted practices to guide post-treatment follow-up. We present clinical and histopathological criteria to advance the prediction of disease outcome in NA-treated breast cancer.
Methods: A retrospective consecutive cohort of 257 NA-treated Finnish breast cancer patients with up to 13-year follow-up and the corresponding tissue samples of pre- and post-NAT breast and metastatic specimen were evaluated for prognostic impacts. All relevant clinical and biomarker characteristics potentially correlated with tumor response to NAT, course of disease, or outcome of breast cancer were included in the statistical analyses.
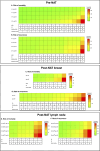
Results: The results highlight the intensified characterization of distinguished prognostic factors and previously overlooked histological features, e.g., mitotic and apoptotic activity. Particularly, decreased PR indicated 3.8-fold (CI 1.9-7.4, p = 0.0001) mortality risk, and a > 10.5-year shorter survival for the majority, > 75% of patients (Q1). Clinically applicable prognostic factors both preceding and following NAT were identified and compiled into heat maps to quantify mortality and recurrence risks. Combinations of risk factors for aggressive disease were exemplified as an interactive tool (bcnatreccalc.utu.fi) to illustrate the spectrum of disease outcomes.
Conclusion: The results emphasize the value of comprehensive evaluation of conventional patient and biomarker characteristics, especially concerning re-assessment of biomarkers, risk-adapted surveillance, and personalized treatment strategies. Future personalized NA-treatment strategies might benefit from models combining risk-adapted surveillance data and post-NAT re-assessed biomarkers.
Keywords: Biomarker; Breast cancer; Neoadjuvant; Prognosis; Risk evaluation.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: All authors declare no financial or non-financial competing interests. Ethical approval: The research was approved by the Regional Ethical Review Board of Turku University Hospital (53/1801/2020) with registry research permits (Turku CRC T186/2020, SATSHP 59/2020, VSHP 30/2020). The research use of tissue materials was granted based on Finnish legislation (the Act on the Medical Use of Human Organs, Tissues and Cells, FINLEX 21a§, 689/2012) by FIMEA (2020/006811 and 2021/003921) and, when feasible, in association with Auria Biobank, Turku, Finland (AB20-4076). The research was performed in accordance with the ethical standards of the World Medical Associations code of ethics ( https://www.wma.net/policies-post/wma-international-code-of-medical-ethics/ ) and the 1964 Helsinki declaration and its later amendments ( https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-researchinvolving-human-subjects/ ). As tissue samples were used retrospectively, the need to obtain informed consent was waived. The interactive tool for estimating the risk of recurrence in neoadjuvant-treated breast cancer patients is based on the current research results. The risk estimate follows the input data given by the user as numbers or Boolean types. The process is totally anonymous, and no sensitive patient data are addressed. Calculation method applies a decision tree method, and the resulting estimate represents cumulated risk in relation to the current cohort. The tool does not use any database enabling open access. Hence, no privacy statement or data protection methods are required to meet the General Data Protection Regulation (GDPR) requirements.
Figures

References
-
- Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, Zackrisson S, Senkus E, Guidelines Committee ESMO (2019) Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 30(8):1194–1220. 10.1093/annonc/mdz173 - PubMed
-
- Cardoso F, Spence D, Mertz S, Corneliussen-James D, Sabelko K, Gralow J, Cardoso MJ, Peccatori F, Paonessa D, Benares A, Sakurai N, Beishon M, Barker SJ, Mayer M (2018) Global analysis of advanced/metastatic breast cancer: decade report (2005–2015). Breast 39:131–138. 10.1016/j.breast.2018.03.002 - PubMed
-
- Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES, Khan SA, Loibl S, Morris EA, Perez A, Regan MM, Spears PA, Sudheendra PK, Symmans WF, Yung RL, Harvey BE, Hershman DL (2021) Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol 39(13):1485–1505. 10.1200/JCO.20.03399 - PMC - PubMed
-
- Loi S (2019) The ESMO clinical practise guidelines for early breast cancer: diagnosis, treatment and follow-up: on the winding road to personalized medicine. Ann Oncol 30(8):1183–1184. 10.1093/annonc/mdz201 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

