Real-World Outcomes After Switch From Aflibercept to Faricimab in Eyes With Diabetic Macular Edema
- PMID: 39739347
- PMCID: PMC11687153
- DOI: 10.1167/iovs.65.14.46
Real-World Outcomes After Switch From Aflibercept to Faricimab in Eyes With Diabetic Macular Edema
Abstract
Purpose: To assess the anatomic and functional outcomes in eyes with diabetic macular edema (DME) switched from intravitreal aflibercept to faricimab in a real-world setting.
Methods: Retrospective, interventional consecutive case series. Patients with DME were switched from aflibercept to faricimab and categorized based on central subfield thickness (CST) 4 weeks after last aflibercept injection into responding DME (rDME, CST reduction >20% or CST ≤ 250 µm) and nonresponding DME (nrDME, CST unchanged or increased). Patients received a loading dose of two monthly faricimab injections followed by a treat-and-extend regimen. Differences in response between rDME and nrDME were analyzed based on injection interval, change in CST, and visual acuity (VA) 12 weeks postswitch.
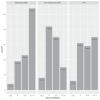
Results: Fifty-two eyes of 40 patients met inclusion criteria (rDME: n = 26, nrDME: n = 26). Baseline and week 12: VA (logMAR) rDME 0.29 ± 0.23 and 0.22 ± 0.28, nrDME 0.42 ± 0.32 and 0.36 ± 0.29; CST (µm) rDME 370 ± 99 and 288 ± 80, nrDME 384 ± 85 and 380 ± 129. After 12 weeks, 54% rDME and 25% nrDME eyes showed a CST decrease of >20% or CST ≤ 250 µm. Forty-six percent rDME and 50% nrDME eyes had a ±20% CST change, 25% of nrDME eyes had a >20% CST increase, and 73% of rDME eyes and 47% of nrDME eyes reached an extended interval of 8 weeks or longer after 12 weeks.
Conclusions: Most DME eyes previously responding or not responding to aflibercept experienced a reduction or stabilization of DME after 12 weeks of faricimab treatment. rDME showed a better anatomic response, and treatment intervals could be extended earlier and longer than nrDME.
Conflict of interest statement
Disclosure:
Figures

References
-
- Jampol LM, Glassman AR, Sun J.. Evaluation and care of patients with diabetic retinopathy. N Engl J Med . 2020; 382(17): 1629–1637. - PubMed
-
- Nguyen QD, Brown DM, Marcus DM, et al. .. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology . 2012; 119(4): 789–801. - PubMed
-
- Heier JS, Korobelnik JF, Brown DM, et al. .. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology . 2016; 123(11): 2376–2385. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

