Disrupted callosal connectivity underlies long-lasting sensory-motor deficits in an NMDA receptor antibody encephalitis mouse model
- PMID: 39739422
- PMCID: PMC11870732
- DOI: 10.1172/JCI173493
Disrupted callosal connectivity underlies long-lasting sensory-motor deficits in an NMDA receptor antibody encephalitis mouse model
Abstract
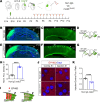
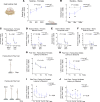
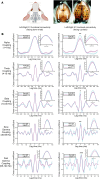
N-methyl-d-aspartate (NMDA) receptor-mediated autoimmune encephalitis (NMDAR-AE) frequently results in persistent sensory-motor deficits, especially in children, yet the underlying mechanisms remain unclear. This study investigated the long-term effects of exposure to a patient-derived GluN1-specific mAb during a critical developmental period (from postnatal day 3 to day 12) in mice. We observed long-lasting sensory-motor deficits characteristic of NMDAR-AE, along with permanent changes in callosal axons within the primary somatosensory cortex (S1) in adulthood, including increased terminal branch complexity. This complexity was associated with paroxysmal recruitment of neurons in S1 in response to callosal stimulation. Particularly during complex motor tasks, mAb3-treated mice exhibited significantly reduced interhemispheric functional connectivity between S1 regions, consistent with pronounced sensory-motor behavioral deficits. These findings suggest that transient exposure to anti-GluN1 mAb during a critical developmental window may lead to irreversible morphological and functional changes in callosal axons, which could significantly impair sensory-motor integration and contribute to long-lasting sensory-motor deficits. Our study establishes a new model of NMDAR-AE and identifies novel cellular and network-level mechanisms underlying persistent sensory-motor deficits in this context. These insights lay the foundation for future research into molecular mechanisms and the development of targeted therapeutic interventions.
Keywords: Autoimmunity; Mouse models; Neurological disorders; Neuroscience.
Figures





Comment in
- Reinvigorating drug development around NGF signaling for pain doi: 10.1172/JCI188251
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

