The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of colorectal cancer, 2024 update
- PMID: 39739441
- PMCID: PMC11947620
- DOI: 10.1002/cac2.12639
The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of colorectal cancer, 2024 update
Abstract
The 2024 updates of the Chinese Society of Clinical Oncology (CSCO) Clinical Guidelines for the diagnosis and treatment of colorectal cancer emphasize standardizing cancer treatment in China, highlighting the latest advancements in evidence-based medicine, healthcare resource access, and precision medicine in oncology. These updates address disparities in epidemiological trends, clinicopathological characteristics, tumor biology, treatment approaches, and drug selection for colorectal cancer patients across diverse regions and backgrounds. Key revisions include adjustments to evidence levels for intensive treatment strategies, updates to regimens for deficient mismatch repair (dMMR)/ microsatellite instability-high (MSI-H) patients, proficient mismatch repair (pMMR)/ microsatellite stability (MSS) patients who have failed standard therapies, and rectal cancer patients with low recurrence risk. Additionally, recommendations for digital rectal examination and DNA polymerase epsilon (POLE)/ DNA polymerase delta 1 (POLD1) gene mutation testing have been strengthened. The 2024 CSCO Guidelines are based on both Chinese and international clinical research, as well as expert consensus, ensuring their relevance and applicability in clinical practice, while maintaining a commitment to scientific rigor, impartiality, and timely updates.
Keywords: Chinese Society of Clinical Oncology (CSCO); adjuvant; chemotherapy; colorectal cancer; diagnosis; immunotherapy; neoadjuvant; radiotherapy; surgery; targeted therapy.
© 2024 The Author(s). Cancer Communications published by John Wiley & Sons Australia, Ltd. on behalf of Sun Yat‐sen University Cancer Center.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
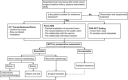
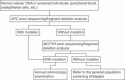
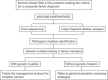
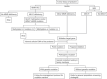
References
-
- Chen H, Lu M, Liu C, Zou S, Du L, Liao X, et al. Comparative Evaluation of Participation and Diagnostic Yield of Colonoscopy vs Fecal Immunochemical Test vs Risk‐Adapted Screening in Colorectal Cancer Screening: Interim Analysis of a Multicenter Randomized Controlled Trial (TARGET‐C). Am J Gastroenterol. 2020;115(8):1264–74. - PubMed
-
- Li QL, Ma XY, Yu LL, Xue F, Ma WL, Yao KY. [Age‐specific detection rates of colorectal neoplasms by colonoscopic screening in high‐incidence rural area]. Zhonghua Zhong Liu Za Zhi. 2013;35(2):154–7. - PubMed
-
- Sung JJ, Ng SC, Chan FK, Chiu HM, Kim HS, Matsuda T, et al. An updated Asia Pacific Consensus Recommendations on colorectal cancer screening. Gut. 2015;64(1):121–32. - PubMed
-
- Tao S, Hoffmeister M, Brenner H. Development and validation of a scoring system to identify individuals at high risk for advanced colorectal neoplasms who should undergo colonoscopy screening. Clin Gastroenterol Hepatol. 2014;12(3):478–85. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

