The role of mechanosignaling in the control of myocardial mass
- PMID: 39739566
- PMCID: PMC12205953
- DOI: 10.1152/ajpheart.00277.2024
The role of mechanosignaling in the control of myocardial mass
Abstract
Regulation of myocardial mass is key for maintaining cardiovascular health. This review highlights the complex and regulatory relationship between mechanosignaling and myocardial mass, influenced by many internal and external factors including hemodynamic and microgravity, respectively. The heart is a dynamic organ constantly adapting to changes in workload (preload and afterload) and mechanical stress exerted on the myocardium, influencing both physiological adaptations and pathological remodeling. Mechanosignaling pathways, such as the mitogen-activated protein kinases (MAPKs) and the phosphoinositide 3-kinases and serine/threonine kinase (PI3K/Akt) pathways, mediate downstream effects on gene expression and play key roles in transducing mechanical cues into biochemical signals, thereby modulating cellular processes, including control of myocardial mass. Dysregulation of these processes can lead to pathological cardiac remodeling, such as hypertrophic cardiomyopathy. Furthermore, recent studies have highlighted the importance of protein quality control mechanisms, such as the ubiquitin-proteasome system, in settings of extreme physiological conditions that alter the heart workload such as pregnancy and microgravity. Overall, this review provides a thorough insight into how mechanical signals are converted into chemical signals to regulate myocardial mass in both healthy and diseased conditions.
Keywords: mechanotransduction; misfolding; proteasome; protein degradation; protein homeostasis.
Conflict of interest statement
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

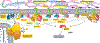


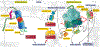

Similar articles
-
Health professionals' experience of teamwork education in acute hospital settings: a systematic review of qualitative literature.JBI Database System Rev Implement Rep. 2016 Apr;14(4):96-137. doi: 10.11124/JBISRIR-2016-1843. JBI Database System Rev Implement Rep. 2016. PMID: 27532314
-
How to Implement Digital Clinical Consultations in UK Maternity Care: the ARM@DA Realist Review.Health Soc Care Deliv Res. 2025 May;13(22):1-77. doi: 10.3310/WQFV7425. Health Soc Care Deliv Res. 2025. PMID: 40417997 Review.
-
Psychological interventions for adults who have sexually offended or are at risk of offending.Cochrane Database Syst Rev. 2012 Dec 12;12(12):CD007507. doi: 10.1002/14651858.CD007507.pub2. Cochrane Database Syst Rev. 2012. PMID: 23235646 Free PMC article.
-
Maternal and neonatal outcomes of elective induction of labor.Evid Rep Technol Assess (Full Rep). 2009 Mar;(176):1-257. Evid Rep Technol Assess (Full Rep). 2009. PMID: 19408970 Free PMC article.
-
Umbelliferone attenuates diabetic sarcopenia by modulating mitochondrial quality and the ubiquitin-proteasome system.Phytomedicine. 2025 Aug;144:156930. doi: 10.1016/j.phymed.2025.156930. Epub 2025 May 31. Phytomedicine. 2025. PMID: 40483791
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

