Reprogramming Dysfunctional Dendritic Cells by a Versatile Catalytic Dual Oxide Antigen-Captured Nanosponge for Remotely Enhancing Lung Metastasis Immunotherapy
- PMID: 39739571
- PMCID: PMC11760334
- DOI: 10.1021/acsnano.4c09525
Reprogramming Dysfunctional Dendritic Cells by a Versatile Catalytic Dual Oxide Antigen-Captured Nanosponge for Remotely Enhancing Lung Metastasis Immunotherapy
Abstract
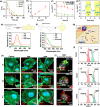
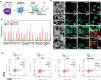
Dendritic cells (DCs) play a crucial role in initiating antitumor immune responses. However, in the tumor environment, dendritic cells often exhibit impaired antigen presentation and adopt an immunosuppressive phenotype, which hinders their function and reduces their ability to efficiently present antigens. Here, a dual catalytic oxide nanosponge (DON) doubling as a remotely boosted catalyst and an inducer of programming DCs to program immune therapy is reported. Intravenous delivery of DON enhances tumor accumulation via the marginated target. At the tumor site, DON incorporates cerium oxide nanozyme (CeO2)-coated iron oxide nanocubes as a peroxide mimicry in cancer cells, promoting sustained ROS generation and depleting intracellular glutathione, i.e., chemodynamic therapy (CDT). Upon high-frequency magnetic field (HFMF) irradiation, CDT accelerates the decomposition of H2O2 and the subsequent production of more reactive oxygen species, known as Kelvin's force laws, which promote the cycle between Fe3+/Fe2+ and Ce3+/Ce4+ in a sustainable active surface. HFMF-boosted catalytic DON promotes tumors to release tumor-associated antigens, including neoantigens and damage-associated molecular patterns. Then, the porous DON acts as an antigen transporter to deliver autologous tumor-associated antigens to program DCs, resulting in sustained immune stimulation. Catalytic DON combined with the immune checkpoint inhibitor (anti-PD1) in lung metastases suppresses tumors and improves survival over 40 days.
Keywords: T cell infiltration; antigen capture; immunotherapy; lung metastasis; nanozymes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
Reprogramming Immunodeficiency in Lung Metastases via PD-L1 siRNA Delivery and Antigen Capture of Nanosponge-Mediated Dendritic Cell Modulation.ACS Nano. 2025 Jul 15;19(27):25134-25153. doi: 10.1021/acsnano.5c05395. Epub 2025 Jul 5. ACS Nano. 2025. PMID: 40616527 Free PMC article.
-
Programmed T cells infiltration into lung metastases with harnessing dendritic cells in cancer immunotherapies by catalytic antigen-capture sponges.J Control Release. 2023 Aug;360:260-273. doi: 10.1016/j.jconrel.2023.06.033. Epub 2023 Jun 29. J Control Release. 2023. PMID: 37364798
-
Vaccine efficacy against primary and metastatic cancer with in vitro-generated CD103+ conventional dendritic cells.J Immunother Cancer. 2020 Apr;8(1):e000474. doi: 10.1136/jitc-2019-000474. J Immunother Cancer. 2020. PMID: 32273347 Free PMC article.
-
Clinically feasible approaches to potentiating cancer cell-based immunotherapies.Hum Vaccin Immunother. 2015;11(4):851-69. doi: 10.1080/21645515.2015.1009814. Hum Vaccin Immunother. 2015. PMID: 25933181 Free PMC article. Review.
-
Dendritic Cell-Based Immunotherapy in Lung Cancer.Front Immunol. 2021 Feb 12;11:620374. doi: 10.3389/fimmu.2020.620374. eCollection 2020. Front Immunol. 2021. PMID: 33679709 Free PMC article. Review.
Cited by
-
Reprogramming Immunodeficiency in Lung Metastases via PD-L1 siRNA Delivery and Antigen Capture of Nanosponge-Mediated Dendritic Cell Modulation.ACS Nano. 2025 Jul 15;19(27):25134-25153. doi: 10.1021/acsnano.5c05395. Epub 2025 Jul 5. ACS Nano. 2025. PMID: 40616527 Free PMC article.
-
An imaging-guided self-amplifying photo-immunotherapeutic nanoparticle for STING pathway activation and enhanced cancer therapy.J Nanobiotechnology. 2025 Jun 21;23(1):459. doi: 10.1186/s12951-025-03536-2. J Nanobiotechnology. 2025. PMID: 40544257 Free PMC article.
-
Hyaluronic acid-tailored prodrug nanoplatforms for efficiently overcoming colorectal cancer chemoresistance and recurrence by synergistic inhibition of cancer cell stemness.J Nanobiotechnology. 2025 Jul 14;23(1):507. doi: 10.1186/s12951-025-03484-x. J Nanobiotechnology. 2025. PMID: 40660238 Free PMC article.
-
Emerging Strategies of Cell and Gene Therapy Targeting Tumor Immune Microenvironment.Clin Cancer Res. 2025 Jun 13;31(12):2294-2308. doi: 10.1158/1078-0432.CCR-24-4308. Clin Cancer Res. 2025. PMID: 40208060 Free PMC article. Review.
-
Leveraging Immunological Properties of Nucleic Acid Nanoparticles to Improve Cancer Therapy.RNA Nanomed. 2025 Apr;2(1):10.59566/isrnn.2025.0201b. doi: 10.59566/isrnn.2025.0201b. RNA Nanomed. 2025. PMID: 40831543 Free PMC article.
References
-
- Shi W.; Feng W.; Li S.; Cui Y.; Liu S.; Jiang H.; Liu Y.; Zhang H. Ferroptosis and Necroptosis Produced Autologous Tumor Cell Lysates Co-Delivering with Combined Immnoadjuvants as Personalized In Situ Nanovaccines Antitumor Immunity. ACS Nano 2023, 17, 14475–14493. 10.1021/acsnano.3c00901. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

