Effects of Graphene Quantum Dots on Renal Fibrosis Through Alleviating Oxidative Stress and Restoring Mitochondrial Membrane Potential
- PMID: 39739624
- PMCID: PMC11904958
- DOI: 10.1002/advs.202410747
Effects of Graphene Quantum Dots on Renal Fibrosis Through Alleviating Oxidative Stress and Restoring Mitochondrial Membrane Potential
Abstract
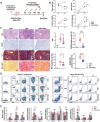
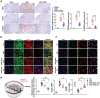
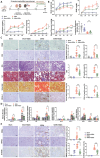
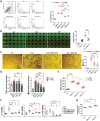
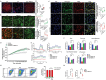
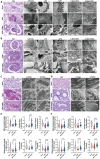
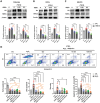
Podocyte injury and proteinuria in glomerular disease are critical indicators of acute kidney injury progression to chronic kidney disease. Renal mitochondrial dysfunction, mediated by intracellular calcium levels and oxidative stress, is a major contributor to podocyte complications. Despite various strategies targeting mitochondria to improve kidney function, effective treatments remain lacking. This study investigates the potential of graphene quantum dots (GQDs) in mitigating renal fibrosis and elucidates their underlying mechanisms. In animal models of Adriamycin-induced nephropathy and 5/6 subtotal nephrectomy, GQDs treatment exhibits anti-inflammatory, anti-fibrotic, and anti-apoptotic effects by restoring podocyte actin structure. These therapeutic benefits are associated with the downregulation of transient receptor potential channel 5 (TRPC5) activity, which is related to kidney fibrosis and mitochondrial dysfunction. In vitro, GQDs suppress TRPC5, enhancing anti-fibrotic and anti-apoptotic effects by lowering calcium levels under oxidative stress and mechanical pressure. Anti-oxidative and anti-senescent effects are also confirmed. Most significantly, transcriptomics and electron microscopy analyses reveal that GQD treatment enhances mitochondrial respiration-related gene profiles and improves mitochondrial cristae morphology. These findings suggest that GQDs are a promising therapeutic nanomaterial for renal cell damage, capable of modulating calcium-dependent apoptosis associated with mitochondrial injury, potentially slowing fibrosis progression.
Keywords: TRPC5; graphene quantum dots; mitochondrial membrane potential; oxidative stress; renal fibrosis.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- a) Zhao C., Song X., Liu Y., Fu Y., Ye L., Wang N., Wang F., Li L., Mohammadniaei M., Zhang M., Zhang Q., Liu J., J. Nanobiotechnol. 2020, 18, 142; - PMC - PubMed
- b) Volarevic V., Paunovic V., Markovic Z., Simovic Markovic B., Misirkic‐Marjanovic M., Todorovic‐Markovic B., Bojic S., Vucicevic L., Jovanovic S., Arsenijevic N., Holclajtner‐Antunovic I., Milosavljevic M., Dramicanin M., Kravic‐Stevovic T., Ciric D., Lukic M. L., Trajkovic V., ACS Nano 2014, 8, 12098; - PubMed
- c) Gao W., Liang Y., Wu D., Deng S., Qiu R., BMC Oral Health 2023, 23, 331. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
