Nano-Armed Limosilactobacillus reuteri for Enhanced Photo-Immunotherapy and Microbiota Tryptophan Metabolism against Colorectal Cancer
- PMID: 39739630
- PMCID: PMC11831460
- DOI: 10.1002/advs.202410011
Nano-Armed Limosilactobacillus reuteri for Enhanced Photo-Immunotherapy and Microbiota Tryptophan Metabolism against Colorectal Cancer
Abstract
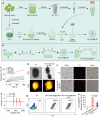
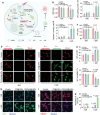
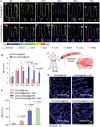
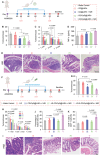
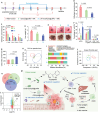
Despite being a groundbreaking approach to treating colorectal cancer (CRC), the efficacy of immunotherapy is significantly compromised by the immunosuppressive tumor microenvironment and dysbiotic intestinal microbiota. Here, leveraging the superior carrying capacity and innate immunity-stimulating property of living bacteria, a nanomedicine-engineered bacterium, LR-S-CD/CpG@LNP, with optical responsiveness, immune-stimulating activity, and the ability to regulate microbiota metabolome is developed. Immunoadjuvant (CpG) and carbon dot (CD) co-loaded plant lipid nanoparticles (CD/CpG@LNPs) are constructed and conjugated to the surface of Limosilactobacillus reuteri (LR) via reactive oxygen species (ROS)-responsive linkers. The inherent photothermal and photodynamic properties of oral CD/CpG@LNPs induce in situ cytotoxic ROS generation and immunogenic cell death of colorectal tumor cells. The generated neoantigens and the released CpG function as a potent in situ vaccine that stimulates the maturation of immature dendritic cells. The mature dendritic cells and metabolites secreted by LR subsequently facilitated the tumor infiltration of cytotoxic T lymphocytes to eradicate colorectal tumors. The further in vivo results demonstrate that the photo-immunotherapy and intestinal microbial metabolite regulation of LR-S-CD/CpG@LNPs collectively suppressed the growth of orthotopic colorectal tumors and their liver metastases, presenting a promising avenue for synergistic treatment of CRC via the oral route.
Keywords: colorectal cancer; engineered bacteria; immunotherapy; oral administration; phototherapy.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
- 82472132/National Natural Science Foundation of China
- 82360110/National Natural Science Foundation of China
- 3240117/National Natural Science Foundation of China
- SWU-XDPY22006/Fundamental Research Funds for the Central Universities
- SWU-KQ22075/Fundamental Research Funds for the Central Universities
- 2205012980212766/Venture & Innovation Support Program for Chongqing Overseas Returnees
- 20212BDH81019/Science and Technology Department of Jiangxi Province
- 20224BAB206073/Science and Technology Department of Jiangxi Province
- KJQN202300223/Science and Technology Research Program of Chongqing Municipal Education Commission
- 2024YFFK0345/Sichuan Science and Technology Program
- CSTB2023NSCQ-MSX0422/Chongqing Science and Technology Foundation
LinkOut - more resources
Full Text Sources
Medical
