Performance of indirect adherence measures for daily oral pre-exposure prophylaxis for HIV among adolescent men who have sex with men and transgender women in Brazil
- PMID: 39739641
- PMCID: PMC11687640
- DOI: 10.1371/journal.pone.0310861
Performance of indirect adherence measures for daily oral pre-exposure prophylaxis for HIV among adolescent men who have sex with men and transgender women in Brazil
Abstract
Background: Consistent monitoring of PrEP adherence with accurate measurement tools at point-of-care could greatly contribute to reaching adolescents with poor adherence. We aimed to assess the performance of indirect adherence measures to oral PrEP among adolescent men who have sex with men (AMSM) and adolescent transgender women (ATGW).
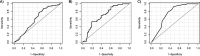
Methods: PrEP15-19 is a prospective, multicenter, PrEP demonstration cohort study that includes AMSM and ATGW aged 15-19 in three Brazilian cities. A diagnostic accuracy study was conducted using tenofovir-diphosphate (TFV-DP) concentrations in dried blood spots as the reference standard, along with three index tests: medication possession ratio (MPR), pill count, and self-report. We calculated the area under the curve (AUC) for protective TFV-DP levels (≥800 fmol/punch) and sensitivity (SE) and specificity (SP) for established cutoff points.
Results: We included 302 samples from 188 participants. Most of participants were AMSM (78.7%), aged 18-19 years (80.3%), and non-whites (72.9%). The AUC was 0.59 for MPR, 0.69 for pill count, and 0.75 for self-report. When combining MPR and self-report, the AUC increased to 0.77. Sensitivity was high for the cutoff points identified by the Youden index, 80% for MPR, 92% for self-report, and 97% for pill count. However, specificities were low 40%, 46%, and 38%, respectively.
Conclusions: Indirect measures were able to discriminate adolescents with good adherence. However, their performance in identifying those with low adherence might be limited, suggesting that it is necessary to initiate adherence interventions when there is no evidence of perfect adherence. Combining measures can provide wider information on adherence.
Copyright: © 2024 Zeballos et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Hosek SG, Landovitz RJ, Kapogiannis B, Siberry GK, Rudy B, Rutledge B, et al. Safety and feasibility of antiretroviral preexposure prophylaxis for adolescent men who have sex with men aged 15 to 17 years in the United States. JAMA Pediatr [Internet]. 2017;171(11):1063–71. Available from: https://jamanetwork.com/ doi: 10.1001/jamapediatrics.2017.2007 - DOI - PMC - PubMed
-
- Schaefer R, Schmidt HMA, Ravasi G, Mozalevskis A, Rewari BB, Lule F, et al. Adoption of guidelines on and use of oral pre-exposure prophylaxis: a global summary and forecasting study. Lancet HIV [Internet]. 2021;8(8):e502–10. Available from: https://www.sciencedirect.com/science/article/pii/S2352301821001272 doi: 10.1016/S2352-3018(21)00127-2 - DOI - PMC - PubMed
-
- Secretaria de Ciência, Tecnologia e Insumos Estratégicos, Ministério da Saúde. Portaria No21, de 25 de maio de 2017. Torna pública a decisão de incorporar o tenofovir associado a entricitabina (TDF/FTC 300/200mg) como profilaxia pré-exposição (PrEP) para [Internet]. Brasil; Available from: https://bvsms.saude.gov.br/bvs/saudelegis/sctie/2017/prt0021_29_05_2017....
-
- Brasil. Protocolo clínico e diretrizes terapêuticas para Profilaxia Pré-exposição (PrEP) de risco à infecção pelo HIV. Ministério da Saúde Brasília; 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous