Hypoxia-induced increase in sphingomyelin synthase 2 aggravates ischemic skeletal muscle inflammation
- PMID: 39739672
- PMCID: PMC11880985
- DOI: 10.1111/febs.17379
Hypoxia-induced increase in sphingomyelin synthase 2 aggravates ischemic skeletal muscle inflammation
Abstract
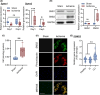
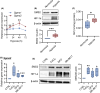
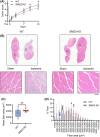
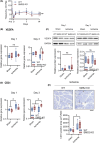
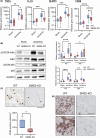
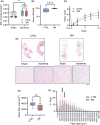
Critical limb ischemia (CLI) is the most advanced stage of peripheral arterial disease, posing a high risk of mortality. Sphingomyelin, a sphingolipid synthesized by sphingomyelin synthases (SMSs) 1 and 2, plays an essential role in signal transduction as a component of lipid rafts. However, the role of sphingomyelin in the inflammation of ischemic skeletal muscles remains unclear. In this study, we analyzed the roles of sphingomyelin and SMSs in CLI-induced myopathy using a mouse hindlimb ischemia model. We observed that hypoxia after CLI triggered an increase in SMS2 levels, thereby elevating sphingomyelin concentrations in ischemic skeletal muscles. The expression of SMS2 and sphingomyelin was induced by hypoxia in C2C12 myotubes and regulated by the prolyl hydroxylase domain enzyme. Additionally, SMS2 deficiency suppressed skeletal muscle inflammation after CLI, attenuated the phosphorylation of inhibitor of κBα (IκBα), and reduced the nuclear translocation of nuclear factor κB (NFκB) p65. Meanwhile, the administration of sphingomyelin hampered skeletal muscle inflammation by inhibiting IκBα phosphorylation and NFκB p65 nuclear translocation and extending inflammation post-CLI. Our results suggest that hypoxia-induced enhancement in SMS2 levels and the consequent increase in sphingomyelin expression levels promote inflammation in ischemic muscle tissues via the NFκB pathway and propose sphingomyelin as a potential therapeutic target in patients with CLI and other hypoxia-related inflammatory diseases.
Keywords: NFκB; critical limb ischemia; hypoxia; sphingomyelin; sphingomyelin synthase 2.
© 2024 The Author(s). The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Sphingomyelin Synthase 2 Inhibition Ameliorates Cerebral Ischemic Reperfusion Injury Through Reducing the Recruitment of Toll-Like Receptor 4 to Lipid Rafts.J Am Heart Assoc. 2019 Nov 19;8(22):e012885. doi: 10.1161/JAHA.119.012885. Epub 2019 Nov 13. J Am Heart Assoc. 2019. PMID: 31718447 Free PMC article.
-
Circadian Dysfunction in the Skeletal Muscle Impairs Limb Perfusion and Muscle Regeneration in Peripheral Artery Disease.Arterioscler Thromb Vasc Biol. 2025 Feb;45(2):e30-e47. doi: 10.1161/ATVBAHA.124.321772. Epub 2024 Dec 5. Arterioscler Thromb Vasc Biol. 2025. PMID: 39633575
-
[Sphingomyelin synthase 2 deficiency decreases atherosclerosis and inhibits inflammation in mice].Sheng Li Xue Bao. 2010 Aug 25;62(4):333-8. Sheng Li Xue Bao. 2010. PMID: 20717634 Chinese.
-
Sphingomyelin synthase 2 activity and liver steatosis: an effect of ceramide-mediated peroxisome proliferator-activated receptor γ2 suppression.Arterioscler Thromb Vasc Biol. 2013 Jul;33(7):1513-20. doi: 10.1161/ATVBAHA.113.301498. Epub 2013 May 2. Arterioscler Thromb Vasc Biol. 2013. PMID: 23640498 Free PMC article.
-
The sphingomyelin synthase family: proteins, diseases, and inhibitors.Biol Chem. 2017 Nov 27;398(12):1319-1325. doi: 10.1515/hsz-2017-0148. Biol Chem. 2017. PMID: 28742512 Review.
References
-
- Olea FD, Locatelli P, Hnatiuk A, De Lorenzi A, Valdivieso L, Rocha E, Ramírez R, Laguens R & Crottogini A (2015) Vascular endothelial growth factor overexpression does not enhance adipose stromal cell‐induced protection on muscle damage in critical limb ischemia. Arterioscler Thromb Vasc Biol 35, 184–188. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

