Immune cell infiltration and modulation of the blood-brain barrier in a guinea pig model of tuberculosis: Observations without evidence of bacterial dissemination to the brain
- PMID: 39739680
- PMCID: PMC11687776
- DOI: 10.1371/journal.pone.0307577
Immune cell infiltration and modulation of the blood-brain barrier in a guinea pig model of tuberculosis: Observations without evidence of bacterial dissemination to the brain
Abstract
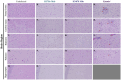
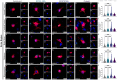
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb) infection, is a chronic inflammatory disease. Although typically associated with inflammation of the lungs and other peripheral tissues, increasing evidence has uncovered neurological consequences attributable to Mtb infection. These include deficits in memory and cognition, increased risk for neurodegenerative disease, and progressive neuropathology. Although the neurological effects of the disease, without CNS infection, have been characterized, the mechanism of neurotoxicity is unknown. We hypothesized that alterations to the blood-brain barrier (BBB) allows peripheral immune cells to enter the brain, initiating a neuroinflammatory response. To test this hypothesis, guinea pigs were exposed by aerosol to a laboratory and a clinical Mtb strain for 15 days. Following Mtb infection, proteins critical to BBB function, including claudin V and collagen IV, are modulated without evidence of bacterial dissemination to the brain. This is correlated with increased contact of astrocytic processes to vessels in the brain, as well as increased expression of the water channel protein aquaporin 4 (AQP4) on endfeet. Upon further investigation, we discovered the potential role of glial reactivity, which is increased following infection with both bacterial strains, in the progression of BBB changes and, ultimately, the permeability of peripheral immune cells into the brain. Through these data, we have obtained a preliminary understanding of the mechanisms of cellular stress in the brain following pulmonary Mtb infection which should be further investigated in future studies.
Copyright: © 2024 Latham et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Organization WH. Global tuberculosis report 2021. 2021 978-92-4-003702-1.
-
- Robertson KR, Oladeji B, Jiang H, Kumwenda J, Supparatpinyo K, Campbell TB, et al. Human Immunodeficiency Virus Type 1 and Tuberculosis Coinfection in Multinational, Resource-limited Settings: Increased Neurological Dysfunction. Clin Infect Dis. 2019;68(10):1739–46. Epub 2018/08/24. doi: 10.1093/cid/ciy718 . - DOI - PMC - PubMed
-
- Frecker MF, Pryse-Philli WEM, Strong HR. Immunological Associations in Familial and Non-Familial Alzheimer Patients and Their Families. Canadian Journal of Neurological Sciences / Journal Canadien des Sciences Neurologiques. 1994;21(2):112–9. Epub 2015/09/18. doi: 10.1017/s0317167100049027 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

