Early life stress shifts critical periods and causes precocious visual cortex development
- PMID: 39739746
- PMCID: PMC11687811
- DOI: 10.1371/journal.pone.0316384
Early life stress shifts critical periods and causes precocious visual cortex development
Abstract
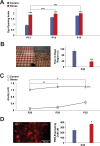
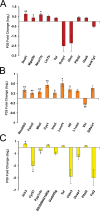
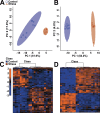
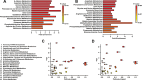
The developing nervous system displays remarkable plasticity in response to sensory stimulation during critical periods of development. Critical periods may also increase the brain's vulnerability to adverse experiences. Here we show that early-life stress (ELS) in mice shifts the timing of critical periods in the visual cortex. ELS induced by animal transportation on postnatal day 12 accelerated the opening and closing of the visual cortex critical period along with earlier maturation of visual acuity. Staining of a molecular correlate that marks the end of critical period plasticity revealed premature emergence of inhibitory perineuronal nets (PNNs) following ELS. ELS also drove lasting changes in visual cortex mRNA expression affecting genes linked to psychiatric disease risk, with hemispheric asymmetries favoring the right side. NMR spectroscopy and a metabolomics approach revealed that ELS was accompanied by activated energy metabolism and protein biosynthesis. Thus, ELS may accelerate visual system development, resulting in premature opening and closing of critical period plasticity. Overall, the data suggest that ELS desynchronizes the orchestrated temporal sequence of regional brain development potentially leading to long-term functional deficiencies. These observations provide new insights into a neurodevelopmental expense to adaptative brain plasticity. These findings also suggest that shipment of laboratory animals during vulnerable developmental ages may result in long lasting phenotypes, introducing critical confounds to the experimental design.
Copyright: © 2024 Poplawski et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Goldman PS, Rakic PT. Impact of the outside world upon the developing primate brain. Perspective from neurobiology. Bull Menninger Clin. 1979;43: 20–28. Available: https://www.ncbi.nlm.nih.gov/pubmed/420959 - PubMed
-
- Lorenz K. The Foundations of Ethology: The principal ideas and discoveries in animal behavior. Simon & Schuster; 1982.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

