Dual-action kinase inhibitors influence p38α MAP kinase dephosphorylation
- PMID: 39739785
- PMCID: PMC11725910
- DOI: 10.1073/pnas.2415150122
Dual-action kinase inhibitors influence p38α MAP kinase dephosphorylation
Abstract
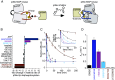
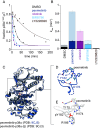
Reversible protein phosphorylation directs essential cellular processes including cell division, cell growth, cell death, inflammation, and differentiation. Because protein phosphorylation drives diverse diseases, kinases and phosphatases have been targets for drug discovery, with some achieving remarkable clinical success. Most protein kinases are activated by phosphorylation of their activation loops, which shifts the conformational equilibrium of the kinase toward the active state. To turn off the kinase, protein phosphatases dephosphorylate these sites, but how the conformation of the dynamic activation loop contributes to dephosphorylation was not known. To answer this, we modulated the activation loop conformational equilibrium of human p38α ΜΑP kinase with existing kinase inhibitors that bind and stabilize specific inactive activation loop conformations. From this, we identified three inhibitors that increase the rate of dephosphorylation of the activation loop phospho-threonine by the PPM serine/threonine phosphatase WIP1. Hence, these compounds are "dual-action" inhibitors that simultaneously block the active site and promote p38α dephosphorylation. Our X-ray crystal structures of phosphorylated p38α bound to the dual-action inhibitors reveal a shared flipped conformation of the activation loop with a fully accessible phospho-threonine. In contrast, our X-ray crystal structure of phosphorylated apo human p38α reveals a different activation loop conformation with an inaccessible phospho-threonine, thereby explaining the increased rate of dephosphorylation upon inhibitor binding. These findings reveal a conformational preference of phosphatases for their targets and suggest a unique approach to achieving improved potency and specificity for therapeutic kinase inhibitors.
Keywords: allostery; drug mechanism; kinase; phosphatase.
Conflict of interest statement
Competing interests statement:D.K. is co-founder of Relay Therapeutics and MOMA Therapeutics. N.B. and E.J.S. are the inventors on a pending patent on a new method for optimizing kinase inhibitors applied for by Brandeis University.
Figures



Update of
-
Dual-Action Kinase Inhibitors Influence p38α MAP Kinase Dephosphorylation.bioRxiv [Preprint]. 2024 Aug 8:2024.05.15.594272. doi: 10.1101/2024.05.15.594272. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2415150122. doi: 10.1073/pnas.2415150122. PMID: 39149408 Free PMC article. Updated. Preprint.
Similar articles
-
Dual-Action Kinase Inhibitors Influence p38α MAP Kinase Dephosphorylation.bioRxiv [Preprint]. 2024 Aug 8:2024.05.15.594272. doi: 10.1101/2024.05.15.594272. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2415150122. doi: 10.1073/pnas.2415150122. PMID: 39149408 Free PMC article. Updated. Preprint.
-
Factors that influence parents' and informal caregivers' views and practices regarding routine childhood vaccination: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2021 Oct 27;10(10):CD013265. doi: 10.1002/14651858.CD013265.pub2. Cochrane Database Syst Rev. 2021. PMID: 34706066 Free PMC article.
-
Roles for TAB1 in regulating the IL-1-dependent phosphorylation of the TAB3 regulatory subunit and activity of the TAK1 complex.Biochem J. 2008 Feb 1;409(3):711-22. doi: 10.1042/BJ20071149. Biochem J. 2008. PMID: 18021073
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Manning G., Whyte D. B., Martinez R., Hunter T., Sudarsanam S., The protein kinase complement of the human genome. Science 298, 1912–1934 (2002). - PubMed
-
- Huse M., Kuriyan J., The conformational plasticity of protein kinases. Cell 109, 275–282 (2002). - PubMed
-
- Endicott J. A., Noble M. E. M., Johnson L. N., The structural basis for control of eukaryotic protein kinases. Annu. Rev. Biochem. 81, 587–613 (2012). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

