A mechanosensitive circuit of FAK, ROCK, and ERK controls biomineral growth and morphology in the sea urchin embryo
- PMID: 39739788
- PMCID: PMC11725891
- DOI: 10.1073/pnas.2408628121
A mechanosensitive circuit of FAK, ROCK, and ERK controls biomineral growth and morphology in the sea urchin embryo
Abstract
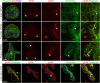
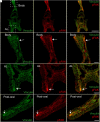
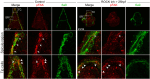
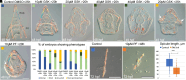
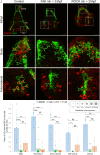
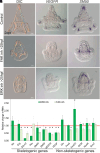
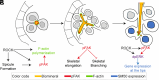
Biomineralization is the utilization of different minerals by a vast array of organisms to form hard tissues and shape them in various forms. Within this diversity, a common feature of all mineralized tissues is their high stiffness, implying that mechanosensing could be commonly used in biomineralization. Yet, the role of mechanosensing in biomineralization is far from clear. Here, we use the sea urchin larval skeletogenesis to investigate the role of substrate stiffness and focal adhesion kinase (FAK) in biomineralization. We demonstrate that substrate stiffness alters spicule morphology and growth, indicating a mechanosensitive response during skeletogenesis. We show that active FAK, F-actin, and vinculin are enriched around the spicules, indicating the formation of focal adhesion complexes and suggesting that the cells sense the mechanical properties of the biomineral. Furthermore, we find that FAK activity is regulated by Rho-associated protein kinase (ROCK) and is crucial for skeletal growth and normal branching. FAK and ROCK activate extracellular signal-regulated kinase (ERK), which regulates skeletogenic gene expression at the tips of the spicules. Thus, the FAK-ROCK-ERK circuit seems to provide essential mechanical feedback on spicule elongation to the skeletogenic gene regulatory network, enabling skeletal growth. Remarkably, the same factors govern mammalian osteoblast differentiation in vitro and pathological calcification in vivo. Thus, this study highlights a common mechanotransduction pathway in biomineralization that was probably independently co-opted across different organisms to shape mineralized structures in metazoans.
Keywords: biomineralization; focal adhesion kinase; gene regulatory networks; mechanotransduction; sea urchin.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

References
-
- Weiner S., Addadi I., Crystallization pathways in biomineralization. Annu. Rev. Mater. Res. 41, 21–40 (2011).
-
- Murdock D. J., Donoghue P. C., Evolutionary origins of animal skeletal biomineralization. Cells Tissues Organs 194, 98–102 (2011). - PubMed
-
- Knoll A. H., Biomineralization and evolutionary history. Rev. Mineral. Geochem. 54, 329–356 (2003).
-
- Murdock D. J. E., The “biomineralization toolkit” and the origin of animal skeletons. Biol. Rev. Camb Philos. Soc. 95, 1372–1392 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

