Aberrant neuronal hyperactivation causes an age-dependent behavioral decline in Caenorhabditis elegans
- PMID: 39739791
- PMCID: PMC11725918
- DOI: 10.1073/pnas.2412391122
Aberrant neuronal hyperactivation causes an age-dependent behavioral decline in Caenorhabditis elegans
Abstract
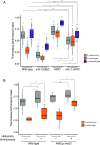
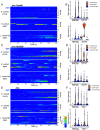
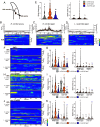
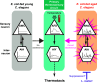
Age-dependent sensory impairment, memory loss, and cognitive decline are generally attributed to neuron loss, synaptic dysfunction, and decreased neuronal activities over time. Concurrently, increased neuronal activity is reported in humans and other organisms during aging. However, it is unclear whether neuronal hyperactivity is the cause of cognitive impairment or a compensatory mechanism of circuit dysfunction. The roundworm Caenorhabditis elegans exhibits age-dependent declines in an associative learning behavior called thermotaxis, in which its temperature preference on a thermal gradient is contingent on food availability during its cultivation. Cell ablation and calcium imaging demonstrate that the major thermosensory circuit consisting of AFD thermosensory neuron and AIY interneuron is relatively intact in aged animals. On the other hand, ablation of either AWC sensory neurons or AIA interneurons ameliorates the age-dependent thermotaxis decline. Both neurons showed spontaneous and stochastic hyperactivity in aged animals, enhanced by reciprocal communication between AWC and AIA via neurotransmitters and neuropeptides. Our findings suggest that AWC and AIA hyperactivity mediates thermotaxis decline in aged animals. Furthermore, dietary modulation could ameliorate age-dependent thermotaxis decline by suppressing neuronal hyperactivity. We propose that aberrantly enhanced, not diminished, neuronal activities can impair the behavior of aged animals.
Keywords: C. elegans; behavioral aging; diet; neuronal hyperactivity; thermotaxis.
Conflict of interest statement
Competing interests statement:The authors declare that they have no competing interests. S.T. is a former employee of MEGMILK SNOW BRAND Co., Ltd.
Figures


References
-
- Petrella J. R., et al. , Cortical deactivation in mild cognitive impairment: High-field-strength functional MR imaging. Radiology. 245, 224–235 (2007). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

