Higher-order transient structures and the principle of dynamic connectivity in membrane signaling
- PMID: 39739805
- PMCID: PMC11725812
- DOI: 10.1073/pnas.2421280121
Higher-order transient structures and the principle of dynamic connectivity in membrane signaling
Abstract
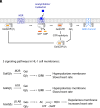
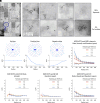
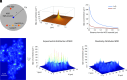
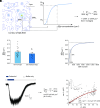
We examine the role of higher-order transient structures (HOTS) in M2R regulation of GIRK channels. Electron microscopic membrane protein location maps show that both proteins form HOTS that exhibit a statistical bias to be near each other. Theoretical calculations and electrophysiological measurements suggest that channel activity is isolated near larger M2R HOTS. By invoking weak interactions that permit transient binding of M2R to M2R and GIRK to GIRK (i-i interactions) and M2R to GIRK (i-j interactions), the distribution patterns and electrophysiological properties of HL-1 cells are replicated in a reaction-diffusion simulation. We propose the principle of dynamic connectivity to explain communication between protein components of a membrane signaling pathway. Dynamic connectivity is mediated by weak, transient interactions between proteins. HOTS created by weak i-i interactions, and statistical biases created by weak i-j interactions promoted by the multivalence of HOTS, are the key elements of dynamic connectivity.
Keywords: GPCR; HOTS; higher-order transient structure; membrane signaling; self-assembly.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E., The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 325, 321–326 (1987). - PubMed
-
- Sakmann B., Noma A., Trautwein W., Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature 303, 250–253 (1983). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

