Molecular basis of hemoglobin binding and heme removal in Corynebacterium diphtheriae
- PMID: 39739808
- PMCID: PMC11725911
- DOI: 10.1073/pnas.2411833122
Molecular basis of hemoglobin binding and heme removal in Corynebacterium diphtheriae
Abstract
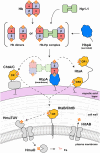
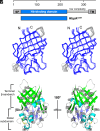
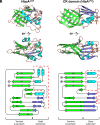
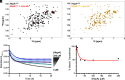
To successfully mount infections, nearly all bacterial pathogens must acquire iron, a key metal cofactor that primarily resides within human hemoglobin. Corynebacterium diphtheriae causes the life-threatening respiratory disease diphtheria and captures hemoglobin for iron scavenging using the surface-displayed receptor HbpA. Here, we show using X-ray crystallography, NMR, and in situ binding measurements that C. diphtheriae selectively captures iron-loaded hemoglobin by partially ensconcing the heme molecules of its α subunits. Quantitative growth and heme release measurements are compatible with C. diphtheriae acquiring heme passively released from hemoglobin's β subunits. We propose a model in which HbpA and heme-binding receptors collectively function on the C. diphtheriae surface to capture hemoglobin and its spontaneously released heme. Acquisition mechanisms that exploit the propensity of hemoglobin's β subunit to release heme likely represent a common strategy used by bacterial pathogens to obtain iron during infections.
Keywords: NMR; X-ray crystallography; bacterial growth; heme capture; hemoglobin.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

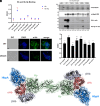
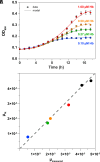
References
-
- Class W. J., Clinical significance of the different forms of the Klebs-Loeffler bacillus. J. Am. Med. Associat., 1019–1020 (1898).
-
- Schmitt M. P., “Iron acquisition and iron-dependent gene expression in Corynebacterium diphtheriae” in Corynebacterium Diphtheriae and Related Toxigenic Species: Genomics, Pathogenicity and Applications, Burkovski A., Ed. (Springer Netherlands, 2014), pp. 95–121.
-
- Kunkle C. A., Schmitt M. P., Analysis of the Corynebacterium diphtheriae DtxR regulon: Identification of a putative siderophore synthesis and transport system that is similar to the yersinia high-pathogenicity island-encoded Yersiniabactin synthesis and uptake system. J. Bacteriol. 185, 6826–6840 (2003). - PMC - PubMed
MeSH terms
Substances
Grants and funding
- T32 GM145388/GM/NIGMS NIH HHS/United States
- T32 AI007323/AI/NIAID NIH HHS/United States
- S10 OD025073/OD/NIH HHS/United States
- R01 AI161828/AI/NIAID NIH HHS/United States
- R01AI161828/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- T32 GM136614/GM/NIGMS NIH HHS/United States
- P30 GM124165/GM/NIGMS NIH HHS/United States
- T90DE030860/HHS | NIH | National Institute of Dental and Craniofacial Research (NIDCR)
- R01 DE025015/DE/NIDCR NIH HHS/United States
- DE025015/HHS | NIH | National Institute of Dental and Craniofacial Research (NIDCR)
- T90 DE030860/DE/NIDCR NIH HHS/United States
- S10 OD016336/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

