Higher-order transient membrane protein structures
- PMID: 39739811
- PMCID: PMC11725870
- DOI: 10.1073/pnas.2421275121
Higher-order transient membrane protein structures
Abstract
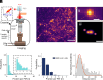
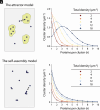
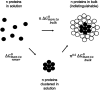
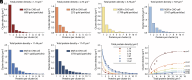
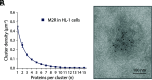
This study shows that five membrane proteins-three GPCRs, an ion channel, and an enzyme-form self-clusters under natural expression levels in a cardiac-derived cell line. The cluster size distributions imply that these proteins self-oligomerize reversibly through weak interactions. When the concentration of the proteins is increased through heterologous expression, the cluster size distributions approach a critical distribution at which point a phase transition occurs, yielding larger bulk phase clusters. A thermodynamic model like that explaining micellization of amphiphiles and lipid membrane formation accounts for this behavior. We propose that many membrane proteins exist as oligomers that form through weak interactions, which we call higher-order transient structures (HOTS). The key characteristics of HOTS are transience, molecular specificity, and a monotonically decreasing size distribution that may become critical at high concentrations. Because molecular specificity invokes self-recognition through protein sequence and structure, we propose that HOTS are genetically encoded supramolecular units.
Keywords: GPCR; HOTS; higher-order transient structure; membrane signaling; self-assembly.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

