Neutrophil extracellular traps have active DNAzymes that promote bactericidal activity
- PMID: 39739822
- PMCID: PMC11797030
- DOI: 10.1093/nar/gkae1262
Neutrophil extracellular traps have active DNAzymes that promote bactericidal activity
Abstract
The mechanisms of bacterial killing by neutrophil extracellular traps (NETs) are unclear. DNA, the largest component of NETs was believed to merely be a scaffold with antimicrobial activity only through the charge of the backbone. Here, we demonstrate for the first time that NETs DNA is beyond a mere scaffold to trap bacteria and it produces hydroxyl free radicals through the spatially concentrated G-quadruplex/hemin DNAzyme complexes, driving bactericidal effects. Immunofluorescence staining showed potential colocalization of G-quadruplex and hemin in extruded NETs DNA, and Amplex UltraRed assay portrayed its peroxidase activity. Proximity labeling of bacteria revealed localized concentration of radicals resulting from NETs bacterial trapping. Ex vivo bactericidal assays revealed that G-quadruplex/hemin DNAzyme is the primary driver of bactericidal activity in NETs. NETs are DNAzymes that may have important biological consequences.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

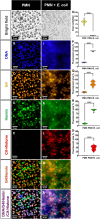


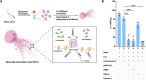
Update of
-
Neutrophil Extracellular Traps have DNAzyme activity that drives bactericidal potential.bioRxiv [Preprint]. 2023 Oct 24:2023.10.23.563618. doi: 10.1101/2023.10.23.563618. bioRxiv. 2023. Update in: Nucleic Acids Res. 2025 Jan 24;53(3):gkae1262. doi: 10.1093/nar/gkae1262. PMID: 37961380 Free PMC article. Updated. Preprint.
Similar articles
-
Neutrophil Extracellular Traps have DNAzyme activity that drives bactericidal potential.bioRxiv [Preprint]. 2023 Oct 24:2023.10.23.563618. doi: 10.1101/2023.10.23.563618. bioRxiv. 2023. Update in: Nucleic Acids Res. 2025 Jan 24;53(3):gkae1262. doi: 10.1093/nar/gkae1262. PMID: 37961380 Free PMC article. Updated. Preprint.
-
Interaction of hemin with quadruplex DNA.J Biol Phys. 2017 Mar;43(1):5-14. doi: 10.1007/s10867-016-9430-7. Epub 2016 Oct 17. J Biol Phys. 2017. PMID: 27752804 Free PMC article.
-
Positive effects of ATP on G-quadruplex-hemin DNAzyme-mediated reactions.Anal Chem. 2010 Jul 15;82(14):6148-53. doi: 10.1021/ac100940v. Anal Chem. 2010. PMID: 20552961
-
Hemin/G-Quadruplex Horseradish Peroxidase-Mimicking DNAzyme: Principle and Biosensing Application.Adv Biochem Eng Biotechnol. 2020;170:85-106. doi: 10.1007/10_2017_37. Adv Biochem Eng Biotechnol. 2020. PMID: 29143069 Review.
-
Bioanalytical Application of Peroxidase-Mimicking DNAzymes: Status and Challenges.Adv Biochem Eng Biotechnol. 2020;170:59-84. doi: 10.1007/10_2017_7. Adv Biochem Eng Biotechnol. 2020. PMID: 28474157 Review.
Cited by
-
Diosmetin Inhibits NETs Formation in Neutrophils Through Regulating Nrf2 Signaling.Thorac Cancer. 2025 Mar;16(6):e70040. doi: 10.1111/1759-7714.70040. Thorac Cancer. 2025. PMID: 40110767 Free PMC article.
-
Tandem ssDNA in neutrophil extracellular traps binds thrombin and regulates immunothrombosis.Proc Natl Acad Sci U S A. 2025 Jul 8;122(27):e2418191122. doi: 10.1073/pnas.2418191122. Epub 2025 Jul 3. Proc Natl Acad Sci U S A. 2025. PMID: 40608679
References
-
- Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A.. Neutrophil extracellular traps kill bacteria. Science. 2004; 303:1532–1535. - PubMed
-
- Azzouz L., Cherry A., Riedl M., Khan M., Pluthero F.G., Kahr W.H.A., Palaniyar N., Licht C.. Relative antibacterial functions of complement and NETs: nETs trap and complement effectively kills bacteria. Mol. Immunol. 2018; 97:71–81. - PubMed
-
- Menegazzi R., Decleva E., Dri P.. Killing by neutrophil extracellular traps: fact or folklore?. Blood. 2012; 119:1214–1216. - PubMed
-
- Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018; 18:134–147. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

