Data mining of adverse drug event signals with Nirmatrelvir/Ritonavir from FAERS
- PMID: 39739861
- PMCID: PMC11687713
- DOI: 10.1371/journal.pone.0316573
Data mining of adverse drug event signals with Nirmatrelvir/Ritonavir from FAERS
Abstract
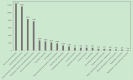
Nirmatrelvir/Ritonavir, acting as an effective agent against COVID-19, has achieved considerable results in clinical studies in terms of drug efficacy. However, there is little research about its medication safety. Based on the FDA adverse event reporting system (FAERS) database, this study aims to mine the adverse reaction signals of the latest major recommended drug Nirmatrelvir/Ritonavir for the antiviral treatment of COVID-19, so as to provide a basis for safe and rational drug use. The reporting odds ratio (ROR) was used to explore the adverse event report data of all COVID-19 emergency use authorization (EUA) products in the FAERS database with the deadline of third quarter of 2023. In the analysis, 135427 adverse drug event (ADE) reports were found, and 35250 ADEs were reported with Nirmatrelvir/Ritonavir as the primary suspected drug, which was involved in multiple system. There was a high signal intensity of dysgeusia (ROR = 72.98), diarrhea (ROR = 3.03) and headache (ROR = 1.25), which was compatible with the adverse reactions recorded in the manual for Nirmatrelvir/Ritonavir. In addition, it was suggested that Nirmatrelvir/Ritonavir might cause pale-colored stools (ROR = 45.53), chromaturia (ROR = 3.07), yellow skin (ROR = 3.62), tongue coating (ROR = 35.55) and other new adverse reactions (not included in the instructions manual for Nirmatrelvir/Ritonavir). The ADEs of Nirmatrelvir/Ritonavir that are not in the instructions and are highly relevant in the real world are supplemented, prompting clinical attention to the ADEs of the drug, and providing a theoretical basis for the safe and effective application of the drug.
Copyright: © 2024 Sun et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The author(s) declare no conflict of interest.
Figures


Similar articles
-
Investigating the Safety Profile of Fast-Track COVID-19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study.Pharmacoepidemiol Drug Saf. 2024 Nov;33(11):e70043. doi: 10.1002/pds.70043. Pharmacoepidemiol Drug Saf. 2024. PMID: 39533148
-
Post-marketing safety concerns with nirmatrelvir: A disproportionality analysis of spontaneous reports submitted to the FDA Adverse Event Reporting System.Br J Clin Pharmacol. 2023 Sep;89(9):2830-2842. doi: 10.1111/bcp.15783. Epub 2023 May 25. Br J Clin Pharmacol. 2023. PMID: 37170890
-
Adverse Events of SARS-CoV-2 Therapy: A Pharmacovigilance Study of the FAERS Database.Ann Pharmacother. 2024 Feb;58(2):105-109. doi: 10.1177/10600280231169256. Epub 2023 May 5. Ann Pharmacother. 2024. PMID: 37144730 Free PMC article.
-
Molnupiravir and Nirmatrelvir-Ritonavir: Oral Coronavirus Disease 2019 Antiviral Drugs.Clin Infect Dis. 2023 Jan 6;76(1):165-171. doi: 10.1093/cid/ciac180. Clin Infect Dis. 2023. PMID: 35245942 Free PMC article. Review.
-
Preclinical discovery and development of nirmatrelvir/ritonavir combinational therapy for the treatment of COVID-19 and the lessons learned from SARS-COV-2 variants.Expert Opin Drug Discov. 2023 Jul-Dec;18(12):1301-1311. doi: 10.1080/17460441.2023.2248879. Epub 2023 Aug 23. Expert Opin Drug Discov. 2023. PMID: 37614103 Review.
Cited by
-
Comparative Analysis of Neuropsychiatric Adverse Reactions Associated with Remdesivir and Nirmatrelvir/Ritonavir in COVID-19 Treatment: Insights from EudraVigilance Data.J Clin Med. 2025 Mar 11;14(6):1886. doi: 10.3390/jcm14061886. J Clin Med. 2025. PMID: 40142695 Free PMC article.
-
Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data.Biomedicines. 2025 Jun 5;13(6):1387. doi: 10.3390/biomedicines13061387. Biomedicines. 2025. PMID: 40564106 Free PMC article.
References
-
- Sun J, Deng X, Chen X, Huang J, Huang S, Li Y, et al.. Incidence of Adverse Drug Reactions in COVID-19 Patients in China: An Active Monitoring Study by Hospital Pharmacovigilance System. Clinical pharmacology and therapeutics. 2020; 108(4): 791–797. Epub 2020/04/24. doi: 10.1002/cpt.1866 . - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

