A replication-incompetent adenoviral vector encoding for HSV-2 gD2 is immunogenic and protective against HSV-2 intravaginal challenge in mice
- PMID: 39739963
- PMCID: PMC11687876
- DOI: 10.1371/journal.pone.0310250
A replication-incompetent adenoviral vector encoding for HSV-2 gD2 is immunogenic and protective against HSV-2 intravaginal challenge in mice
Abstract
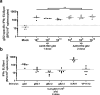
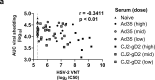
Herpes Simplex virus (HSV) is the cause of genital herpes and no prophylactic treatment is currently available. Replication-incompetent adenoviral vectors are potent inducers of humoral and cellular immune responses in humans. We have designed an adenoviral vector type 35 (Ad35)-based vaccine encoding the HSV-2 major surface antigen gD2 (Ad35.HSV.gD2). Immunization of mice with Ad35.HSV.gD2 elicited virus neutralizing antibody titers (VNT) and cellular responses against HSV-2 and HSV-1. While immunity was lower than for CJ2-gD2, both vaccines showed 100% survival against intravaginal challenge with HSV-2 G strain and a strong inverse correlation was observed between HSV-2 infection (as measured by viral shedding) and VNT. A combination of Ad35.HSV.gD2 with Ad35 encoding for gB2 (Ad35.HSV.gB2) resulted in increased VNT and lower infection, compared with Ad35.HSV.gD2 alone. Transfer of immune serum into naïve BALB/c mice before intravaginal challenge confirmed the role of antibodies in the protection of mice against infection although other immune factors may play a role as well.
Copyright: © 2024 Rossetti et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: M.V., E.H., J.T., R.Z., H.S. and E.S. are employees of Janssen Infectious Diseases & Vaccines and may own stock or stock options in Johnson & Johnson, its parent company. E.R is currently an employee of Immunocore Ltd. and reports no competing interest. E.H. is currently an employee of Leiden University Medical Center and reports no competing interest. H.S. is currently an employee of Valneva SE and reports no competing interest. F.Y. is currently an employee of SalioGen and reports no competing interest. E.S is currently an employee of Genmab and reports no competing interest. This does not alter our adherence to PLOS ONE policies on sharing data and materials. There are no patents, products in development or marketed products associated with this research to declare.
Figures





References
-
- Roberts C. Genital herpes in young adults: changing sexual behaviours, epidemiology and management. Herpes. 2005;12(1):10–4. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

