Comprehensive characterization of MCL-1 in patients with colorectal cancer: Expression, molecular profiles, and outcomes
- PMID: 39740007
- PMCID: PMC11826129
- DOI: 10.1002/ijc.35304
Comprehensive characterization of MCL-1 in patients with colorectal cancer: Expression, molecular profiles, and outcomes
Abstract
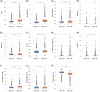
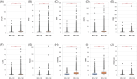
Myeloid cell leukemia 1 (MCL-1) is a member of the B-cell lymphoma 2 protein family and has anti-apoptotic functions. Deregulation of MCL-1 has been reported in several cancers, including lung and breast cancer. In the present study, the association of MCL-1 expression with molecular features in colorectal cancer (CRC) has been highlighted. CRC samples from Caris Life Sciences (Phoenix, AZ) were analyzed using NextGen DNA sequencing, whole transcriptome sequencing, whole exome sequencing, and immunohistochemistry (IHC); and stratified based on MCL-1 expression as top quartile MCL-1high (Q4) and bottom quartile MCL-1low (Q1). Immune cell infiltration (CI) in the tumor microenvironment (TME) was measured using RNA deconvolution analysis (QuanTIseq). MCL-1high tumors were associated with an increased rate of programmed death ligand 1 IHC, higher T cell-inflamed signature, interferon score, microsatellite instability-high and tumor mutational burden-high status. MCL-1high was associated with higher mutation rates of BCOR, TP53, KMT2D, ASXL1, KDM6A, ATM, MSH6, SPEN, KRAS, STK11, GNAS, RNF43, and lower mutation rates of CDKN1B, NRAS, and APC, and copy number amplifications in several genes. MCL-1high TME had higher CI of M1 and M2 macrophages, B cells, natural killer cells, neutrophils, and T-regulatory cells infiltration, and lower CI of myeloid dendritic cells. Higher MCL-1 expression is significantly associated with favorable clinical outcomes in CRC cohorts. Our data showed a strong correlation between MCL-1 and distinct immune biomarkers and TME CI in CRC. Our findings suggest MCL-1 is a potential modulator of antitumor immunity, TME, and biomarker in CRC.
Keywords: MCL‐1; colorectal cancer; infiltration; mutations; rectal tumors; tumor microenvironment.
© 2024 The Author(s). International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
Yasmine Baca, Joanne Xiu, and Alex Farrell are employees of Caris Life Sciences, Phoenix, AZ, USA. Benjamin A. Weinberg is a consultant for Caris Life Sciences; no other relevant disclosures. Emil Lou reports support from the University of Minnesota Clinical Center for the Study of Pancreatic Disease, part of The Chronic Pancreatitis Diabetes Pancreatic Cancer research (CPDPC) consortium funded by the NIDDK (5U01DK126300‐03), and research grants from the American Cancer Society (RSG‐22‐022‐01‐CDP) 2022–2026, and the Minnesota Ovarian Cancer Alliance in 2019, 2021, and 2022; American Association for Cancer Research (2019 AACR‐Novocure Tumor‐Treating Fields Research Grant, grant number 1‐60‐62‐LOU); The Randy Shaver Cancer Research and Community Fund; honorarium and travel expenses for a research talk at GlaxoSmithKline in 2016; honoraria and travel expenses for lab‐based research talks 2018‐21, and equipment for laboratory‐based research 2018‐present, Novocure, Ltd.; honorarium for panel discussion organized by Antidote Education for a Continuing Medical Education (CME) module on diagnostics and treatment of HER2+ gastric and CRCs, funded by Daiichi‐Sankyo, 2021 (honorarium donated to lab); compensation for scientific review of proposed printed content, Elsevier Publishing and Johns Hopkins Press; consultant, Nomocan Pharmaceuticals (no financial compensation); Scientific Advisory Board Member, Minnetronix, LLC, 2018–2019 (no financial compensation); consultant and speaker honorarium, Boston Scientific US, 2019. Institutional Principal Investigator for clinical trials sponsored by Celgene, Novocure, Ltd., Intima Bioscience, Inc., the National Cancer Institute, and University of Minnesota membership in the Caris Life Sciences Precision Oncology Alliance (no financial compensation), and thanks the following groups for donations in support of cancer research: The Mu Sigma Chapter of the Phi Gamma Delta Fraternity, University of Minnesota (FIJI); the Litman Family Fund for Cancer Research; Dick and Lynnae Koats; Ms. Patricia Johnson. Anthony F. Shields reports receiving research support and being on the speaker's bureau from Caris Life Sciences, Phoenix, AZ, USA. Richard M. Goldberg reports receiving honoraria from consultant/advisory board membership from AbbVie, Artemida, Bayer, Eisai, G1 Therapeutics, GeneCentric, GSK, Haystack Oncology, Focal Medical Inc., Merck, Modulation Therapeutics, Sorrento, Taiho, Takeda, UpToDate, and Valar Labs. John L. Marshall reports being an advisor/consultant for AZ, Merck, Bayer, Taiho, Pfizer, Takeda, Astellas. Heinz‐Josef Lenz reports receiving honoraria from consultant/advisory board membership from Bayer, Genentech, Roche, Merck, Merck KG, Oncocyte, Fulgent, G1 Therapeutics, 3T Biosciences, Jazz Therapeutics, Protagonist. All authors' disclosures have been mentioned above. The other authors declare no potential conflicts of interest.
Figures



References
-
- Mittal P, Singh S, Sinha R, Shrivastava A, Singh A, Singh IK. Myeloid cell leukemia 1 (MCL‐1): structural characteristics and application in cancer therapy. Int J Biol Macromol. 2021;187:999‐1018. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

