Turn TRAIL Into Better Anticancer Therapeutic Through TRAIL Fusion Proteins
- PMID: 39740038
- PMCID: PMC11683677
- DOI: 10.1002/cam4.70517
Turn TRAIL Into Better Anticancer Therapeutic Through TRAIL Fusion Proteins
Abstract
Background: TNF-related apoptosis-inducing ligand (TRAIL) belongs to the tumor necrosis factor superfamily. TRAIL selectively induces apoptosis in tumor cells while sparing normal cells, which makes it an attractive candidate for cancer therapy. Recombinant soluble TRAIL and agonistic antibodies against TRAIL receptors have demonstrated safety and tolerability in clinical trials. However, they have failed to exhibit expected clinical efficacy. Consequently, extensive research has focused on optimizing TRAIL-based therapies, with one of the most common approaches being the construction of TRAIL fusion proteins.
Methods: An extensive literature search was conducted to identify studies published over the past three decades related to TRAIL fusion proteins. These various TRAIL fusion strategies were categorized based on their effects achieved.
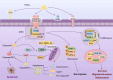
Results: The main fusion strategies for TRAIL include: 1. Construction of stable TRAIL trimers; 2. Enhancing the polymerization capacity of soluble TRAIL; 3. Increasing the accumulation of TRAIL at tumor sites by fusing with antibody fragments or peptides; 4. Decorating immune cells with TRAIL; 5. Prolonging the half-life of TRAIL in vivo; 6. Sensitizing cancer cells to overcome resistance to TRAIL treatment.
Conclusion: This work focuses on the progress in recombinant TRAIL fusion proteins and aims to provide more rational and effective fusion strategies to enhance the efficacy of recombinant soluble TRAIL, facilitating its translation from bench to bedside as an effective anti-cancer therapeutic.
Keywords: TRAIL; apoptosis; cancer; fusion protein; resistance.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Engineered adenovirus fiber shaft fusion homotrimer of soluble TRAIL with enhanced stability and antitumor activity.Cell Death Dis. 2016 Jun 23;7(6):e2274. doi: 10.1038/cddis.2016.177. Cell Death Dis. 2016. PMID: 27336718 Free PMC article.
-
Novel engineered TRAIL-based chimeric protein strongly inhibits tumor growth and bypasses TRAIL resistance.Int J Cancer. 2020 Aug 15;147(4):1117-1130. doi: 10.1002/ijc.32845. Epub 2020 Jan 24. Int J Cancer. 2020. PMID: 31863596
-
Fn14·TRAIL fusion protein is oligomerized by TWEAK into a superefficient TRAIL analog.Cancer Lett. 2017 Aug 1;400:99-109. doi: 10.1016/j.canlet.2017.04.026. Epub 2017 Apr 25. Cancer Lett. 2017. PMID: 28455246
-
TRAIL receptor signalling and modulation: Are we on the right TRAIL?Cancer Treat Rev. 2009 May;35(3):280-8. doi: 10.1016/j.ctrv.2008.11.006. Epub 2008 Dec 30. Cancer Treat Rev. 2009. PMID: 19117685 Review.
-
Onto better TRAILs for cancer treatment.Cell Death Differ. 2016 May;23(5):733-47. doi: 10.1038/cdd.2015.174. Epub 2016 Mar 4. Cell Death Differ. 2016. PMID: 26943322 Free PMC article. Review.
Cited by
-
The role of macrophage polarization in ovarian cancer: from molecular mechanism to therapeutic potentials.Front Immunol. 2025 Apr 22;16:1543096. doi: 10.3389/fimmu.2025.1543096. eCollection 2025. Front Immunol. 2025. PMID: 40330466 Free PMC article. Review.
References
-
- Dianat‐Moghadam H., Heidarifard M., Mahari A., et al., “TRAIL in Oncology: From Recombinant TRAIL to Nano‐ and Self‐Targeted TRAIL‐Based Therapies,” Pharmacological Research 155 (2020): 104716. - PubMed
-
- Thapa B., Kc R., and Uludag H., “TRAIL Therapy and Prospective Developments for Cancer Treatment,” Journal of Controlled Release 326 (2020): 335–349. - PubMed
-
- Takeda K., Hayakawa Y., Smyth M. J., et al., “Involvement of Tumor Necrosis Factor‐Related Apoptosis‐Inducing Ligand in Surveillance of Tumor Metastasis by Liver Natural Killer Cells,” Nature Medicine 7 (2001): 94–100. - PubMed
-
- Bodmer J. L., Meier P., Tschopp J., and Schneider P., “Cysteine 230 Is Essential for the Structure and Activity of the Cytotoxic Ligand TRAIL,” Journal of Biological Chemistry 275 (2000): 20632–20637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous