Global Prevalence and Subtype Distribution of Blastocystis sp. in Rodent Populations: A Systematic Review and Meta-Analysis
- PMID: 39740090
- PMCID: PMC11683779
- DOI: 10.1002/vms3.70178
Global Prevalence and Subtype Distribution of Blastocystis sp. in Rodent Populations: A Systematic Review and Meta-Analysis
Abstract
Background: The present systematic review and meta-analysis aimed to gather and analyse global data on the prevalence, subtypes (STs) distribution and zoonotic potential of Blastocystis sp. in rodents.
Methods: A systematic literature search was performed across multiple databases (PubMed, Scopus, Web of Science and ProQuest) for studies published by 23 July 2024. The analysis included 34 studies/78 datasets, comprising 5661 samples from various rodent species across 15 countries. Statistical analyses were performed using comprehensive meta-analysis (CMA) software, employing a random-effects model to estimate pooled prevalence and 95% confidence intervals (CIs) and the I2 index for assessing heterogeneity.
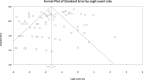
Results: This review found that 16% (95% CI: 12.6%-20.2%) of rodents worldwide were infected with Blastocystis sp. Voles and squirrels exhibited the highest infection rates at 29.8% (95% CI: 14.7%-51%) and 28.8% (95% CI: 14.4%-49.2%), whereas civets and porcupines had the lowest rates at 9.5% (95% CI: 6.6%-13.6%) and 7.1% (95% CI: 3.3%-14.7%), respectively. The findings indicated that rodents can host various Blastocystis sp. STs (ST1-ST8, ST10, ST13, ST15, ST17), with several (ST1-ST8 and ST10) having zoonotic potential. Globally, ST4, ST5, ST1 and ST3 were the most commonly reported STs in rodents. China and the UK showed the highest ST diversity in rodents, with 10 (ST1-ST7, ST10, ST13, ST17) and 7 (ST1-ST5, ST10, ST15) distinct STs, respectively. ST6, ST7 and ST13 were unique to China, whereas ST15 was found only in the United Kingdom. Squirrels, rats, mice and voles had the highest ST diversity of Blastocystis sp., with 8, 7, 5 and 5 distinct STs, respectively. Notably, ST6 and ST13 were unique to squirrels, ST7 only appeared in rats, and ST15 was found only in voles. Most ST1, ST3-ST5 and ST17 came from Asia. ST6, ST7 and ST13 were also isolated there, whereas ST15 was only found in Europe. ST17 was reported in Africa, ST4 and ST17 in North America, and ST1-ST3 and ST8 in South America.
Conclusions: This review emphasizes the widespread presence of Blastocystis sp. in rodent populations globally, underscoring the need for continued surveillance and research into its zoonotic potential.
Keywords: Blastocystis sp; epidemiology; meta‐analysis; rodents; subtypes; systematic review.
© 2024 The Author(s). Veterinary Medicine and Science published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- AbuOdeh, R. , Ezzedine S., Madkour M., et al. 2019. “Molecular Subtyping of Blastocystis From Diverse Animals in the United Arab Emirates.” Protist 170: 125679. - PubMed
-
- Alfellani, M. A. , Taner‐Mulla D., Jacob A. S., et al. 2013. “Genetic Diversity of Blastocystis in Livestock and Zoo Animals.” Protist 164: 497–509. - PubMed
-
- Asghari, A. , Sadeghipour Z., Hassanipour S., et al. 2021. “Association Between Blastocystis Sp. Infection and Immunocompromised Patients: A Systematic Review and Meta‐Analysis.” Environmental Science and Pollution Research 28: 60308–60328. - PubMed
-
- Asghari, A. , Sadrebazzaz A., Shamsi L., and Shams M.. 2021. “Global Prevalence, Subtypes Distribution, Zoonotic Potential, and Associated Risk Factors of Blastocystis Sp. in Domestic Pigs (Sus domesticus) and Wild Boars (Sus scrofa): A Systematic Review and Meta‐Analysis.” Microbial Pathogenesis 160: 105183. - PubMed
-
- Bastaminejad, S. , Eskandari P., Mohammadi M. R., et al. 2024. “Identification of Blastocystis Spp. In Urban Rodents of Different Districts in Southwestern Iran: Subtype Distribution and Possible Zoonotic Potential.” Acta Parasitologica 69: 922–928. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

