Roles of blood monocytes carrying TREM2R47H mutation in pathogenesis of Alzheimer's disease and its therapeutic potential in APP/PS1 mice
- PMID: 39740209
- PMCID: PMC11848385
- DOI: 10.1002/alz.14402
Roles of blood monocytes carrying TREM2R47H mutation in pathogenesis of Alzheimer's disease and its therapeutic potential in APP/PS1 mice
Abstract
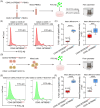
Introduction: The triggering receptor expressed on myeloid cells 2 (TREM2) arginine-47-histidine (R47H) mutation is a significant risk for Alzheimer's disease (AD) with unclear mechanisms. Previous studies focused on microglial amyloid-β (Aβ) phagocytosis with less attention on the impact of TREM2R47H mutation on blood monocytes.
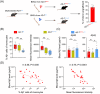
Methods: Bone marrow transplantation (BMT) models were used to assess the contribution of blood monocytes carrying TREM2R47H mutation to AD.
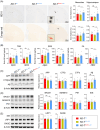
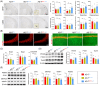
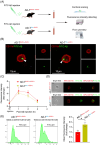
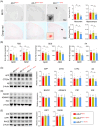
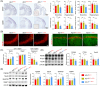
Results: Aβ phagocytosis was compromised in mouse monocytes carrying the TREM2R47H mutation. Transplantation of bone marrow cells (BMCs) carrying TREM2R47H mutation increased cerebral Aβ burden and aggravated AD-type pathologies. Moreover, the replacement of TREM2R47H-BMCs restored monocytic Aβ phagocytosis, lowered Aβ levels in the blood and brain, and improved cognitive function.
Discussion: Our study reveals that blood monocytes carrying the TREM2R47H mutation substantially contribute to the pathogenesis of AD, and correcting the TREM2R47H mutation in BMCs would be a potential therapeutic approach for those carrying this mutation.
Highlights: TREM2R47H mutation compromises the Aβ phagocytosis of blood monocytes. Blood monocytes carrying TREM2R47H mutation contribute substantially to AD pathogenesis. Correction of the TREM2R47H mutation in bone marrow cells ameliorates AD pathologies and cognitive impairments.
Keywords: Alzheimer's disease; Aβ; TREM2R47H mutation; bone marrow transplantation; monocytes; phagocytosis.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare that they have no competing interests. Author disclosures are available in the supporting information.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

