Carbon Monoxide-Releasing Activity of Plant Flavonoids
- PMID: 39740217
- PMCID: PMC11741109
- DOI: 10.1021/acs.jafc.4c09069
Carbon Monoxide-Releasing Activity of Plant Flavonoids
Abstract
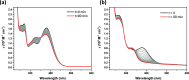
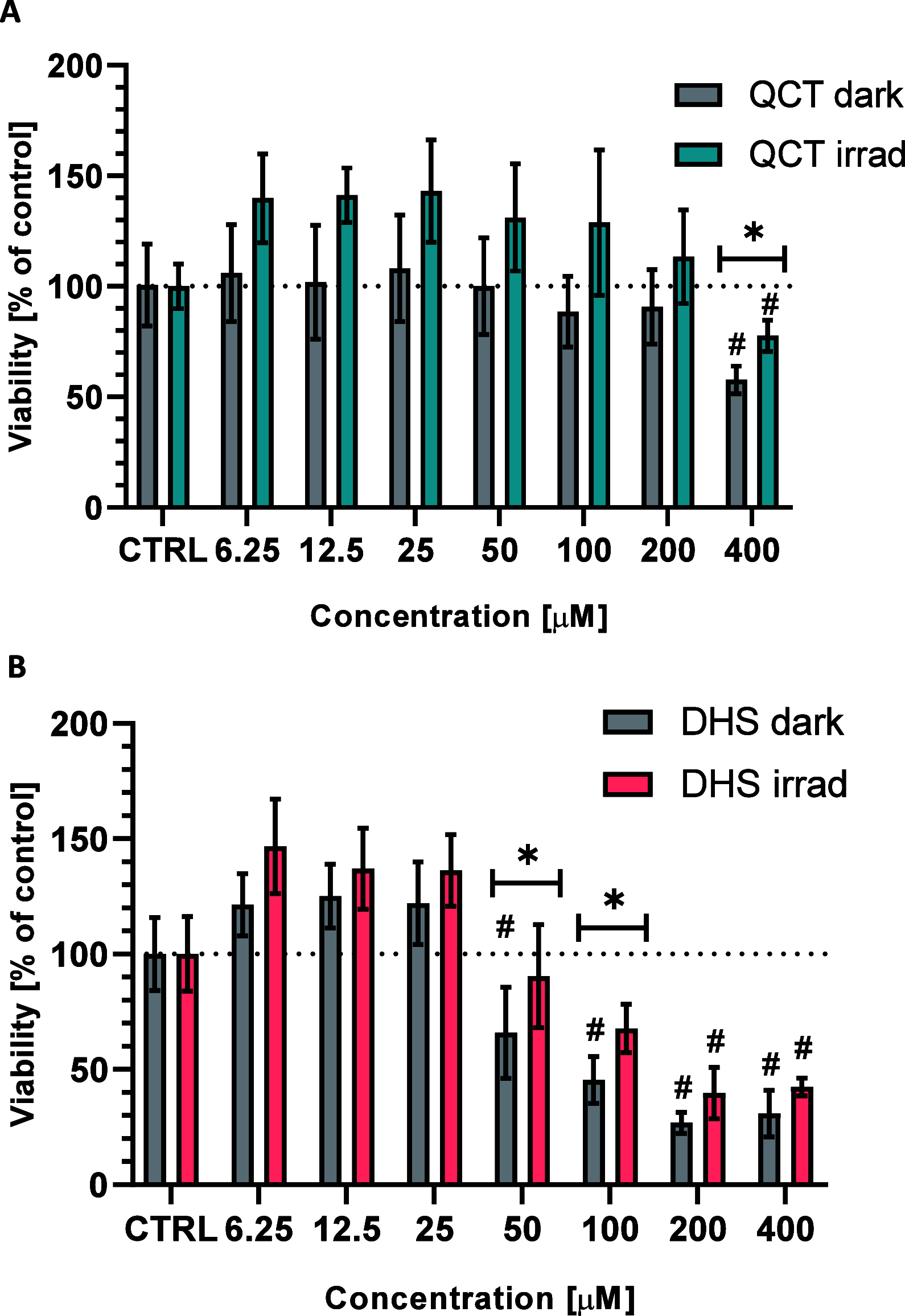
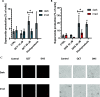
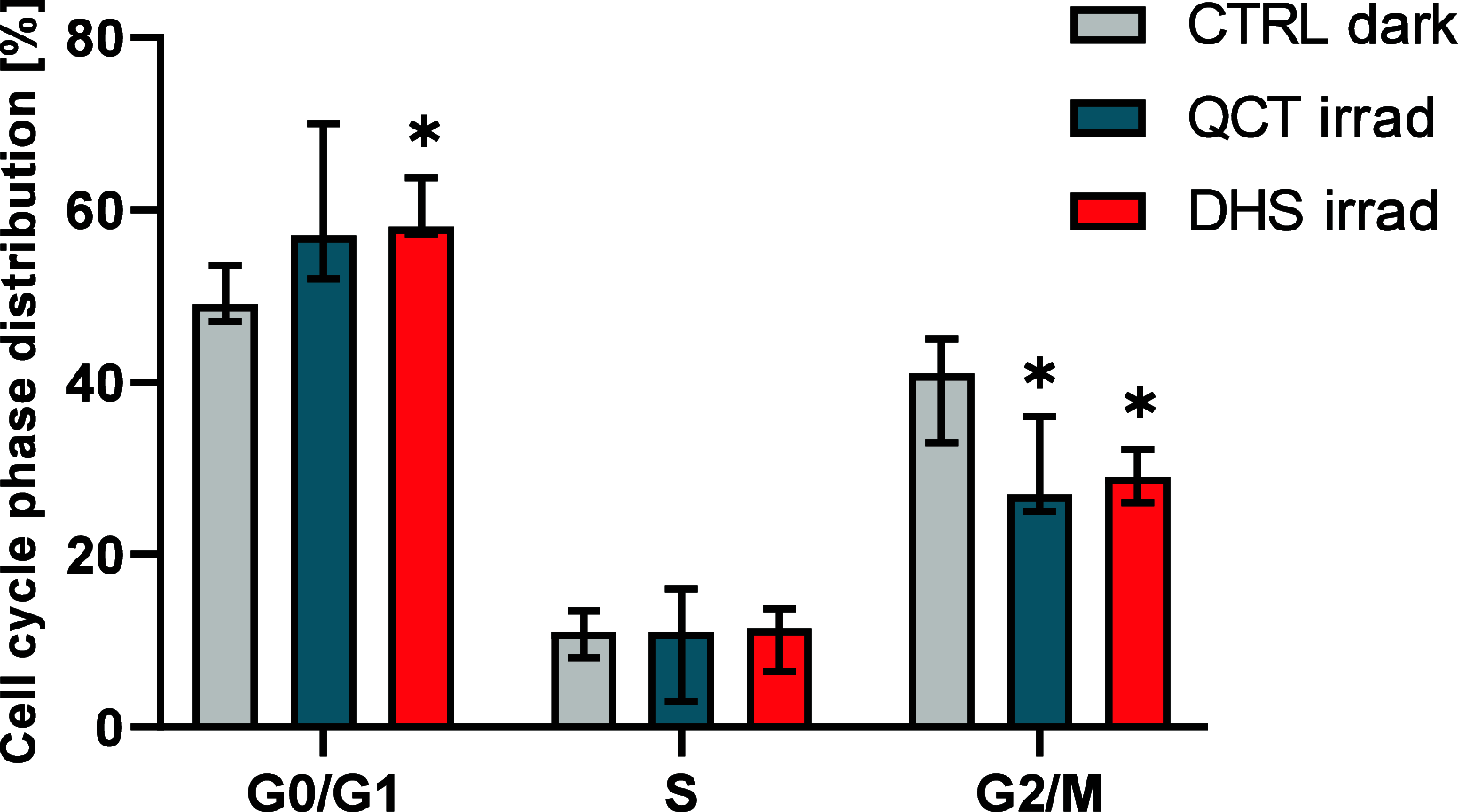
Flavonoids are naturally occurring compounds found in fruits, vegetables, and other plant-based foods, and they are known for their health benefits, such as UV protection, antioxidant, anti-inflammatory, and antiproliferative properties. This study investigates whether flavonoids, such as quercetin and 2,3-dehydrosilybin, can act as photoactivatable carbon monoxide (CO)-releasing molecules under physiological conditions. CO has been recently recognized as an important signaling molecule. Here, we show that upon direct irradiation, CO was released from both flavonoids in PBS with chemical yields of up to 0.23 equiv, which increased to almost unity by sensitized photooxygenation involving singlet oxygen. Photoreleased CO reduced cellular toxicity caused by high flavonol concentrations, partially restored mitochondrial respiration, reduced superoxide production induced by rotenone and high flavonol levels, and influenced the G0/G1 and G2/M phases of the cell cycle, showing antiproliferative effects. The findings highlight the potential of quercetin and 2,3-dehydrosilybin as CO-photoreleasing molecules with chemopreventive and therapeutic implications in human pathology and suggest their possible roles in plant biology.
Keywords: 2,3-dehydrosilybin; carbon monoxide; cell cycle; mitochondrial respiration; oxidative stress; photoCORM; photoinduced release; quercetin.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
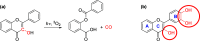




References
-
- Tapas A. R.; Sakarkar D. M.; Kakde R. B. Flavonoids as nutraceuticals: A review. Trop. J. Pharm. Res. 2008, 7 (3), 1089–1099. 10.4314/tjpr.v7i3.14693. - DOI
-
- Pradhan S. C.; Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J. Med. Res. 2006, 124 (5), 491–504. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

