An α7 nicotinic and GABAB receptor-mediated pathway controls acetylcholine release in the tripartite neuromuscular junction
- PMID: 39740234
- PMCID: PMC11737540
- DOI: 10.1113/JP287243
An α7 nicotinic and GABAB receptor-mediated pathway controls acetylcholine release in the tripartite neuromuscular junction
Abstract
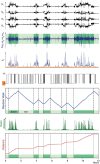
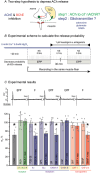
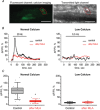
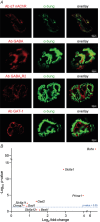
Terminal Schwann cells (TSCs) are capable of regulating acetylcholine (ACh) release at the neuromuscular junction (NMJ). We have identified GABA as a gliotransmitter at mouse NMJs. When ACh activates α7 nicotinic ACh receptor (nAChRs) on TSCs, GABA is released and activates GABAB receptors on the nerve terminal that subsequently reduce ACh release. Indeed, specific deletion of the α7 nAChR in TSCs or inhibition of the metabotropic GABAB receptor prevents the reduction in the quantal content of the end-plate potential induced by cholinesterase inhibitors. The α7/GABAB receptor-mediated pathway is activated when ACh that escapes from collagen Q (ColQ) anchored AChE in the synaptic cleft and from PRiMA-anchored butyrylcholinesterase on the TSC activates α7 nAChRs on the TSC. Consequently, prolonged tetanic stimulation of isolated muscle activates the α7/GABAB receptor pathway, which reduces post-tetanic ACh release. When AChE levels are low in neonatal mice, the α7/GABAB receptor-mediated pathway decreases ACh release and reduces ex vivo muscle fatigue. For ColQ-deficient mice where AChE is not clustered, the decrease in AСh release following activation of this pathway contributes to mouse fatigue in vivo. KEY POINTS: Acetylcholine (ACh) released from the nerve terminal at the neuromuscular junction (NMJ) can activate α7 nicotinic ACh receptor (nAChR) on terminal Schwann cells, releasing gamma-aminobutyric acid (GABA) that activates metabotropic GABAB receptors on the nerve terminal which then reduces further ACh release from the nerve. At the mature NMJ, before reaching α7 nAChRs on terminal Schwann cells ACh is normally hydrolyzed by AChE clustered in the synaptic cleft and by BChE anchored to the TSC. ACh can activate the α7/GABAB receptor-mediated pathway and depress subsequent ACh release when AChE at the NMJ is low, either during development or in congenital myasthenic syndrome. In the latter case, this pathway contributes to muscle fatigue.
Keywords: acetylcholine; acetylcholinesterase; aminobutyric acid; neuromuscular junction.
© 2024 The Author(s). The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
