Adipocyte-derived small extracellular vesicles exacerbate diabetic ischemic heart injury by promoting oxidative stress and mitochondrial-mediated cardiomyocyte apoptosis
- PMID: 39740363
- PMCID: PMC11750569
- DOI: 10.1016/j.redox.2024.103443
Adipocyte-derived small extracellular vesicles exacerbate diabetic ischemic heart injury by promoting oxidative stress and mitochondrial-mediated cardiomyocyte apoptosis
Abstract
Background: Diabetes increases ischemic heart injury via incompletely understood mechanisms. We recently reported that diabetic adipocytes-derived small extracellular vesicles (sEV) exacerbate myocardial reperfusion (MI/R) injury by promoting cardiomyocyte apoptosis. Combining in vitro mechanistic investigation and in vivo proof-concept demonstration, we determined the underlying molecular mechanism responsible for diabetic sEV-induced cardiomyocyte apoptosis after MI/R.
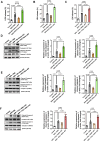
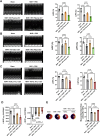
Methods and results: Adult mice were fed a high-fat diet (HFD) for 12 weeks. sEV were isolated from plasma or epididymal adipose tissue. HFD significantly increased the number and size of plasma- and adipocyte-derived sEV. Intramyocardial injection of an equal number of diabetic plasma sEV in nondiabetic hearts significantly increased cardiac apoptosis and exacerbated MI/R-induced cardiac dysfunction. Diabetic plasma sEV significantly activated cardiac caspase 9 but not caspase 8, suggesting that diabetic sEV induces cardiac apoptosis via the mitochondrial pathway. These pathologic alterations were phenotyped by intramyocardial injection of sEV isolated from diabetic adipocytes or HGHL-challenged 3T3L1 adipocytes. To obtain direct evidence that diabetic sEV promotes cardiomyocyte apoptotic cell death, isolated neonatal rat ventricular cardiomyocytes (NRVMs) were treated with sEV and subjected to simulated ischemia/reperfusion (SI/R). Treatment of cardiomyocytes with sEV from diabetic plasma, diabetic adipocytes, or HGHL-challenged 3T3L1 adipocytes significantly enhanced SI/R-induced apoptosis and reduced cell viability. These pathologic effects were replicated by a miR-130b-3p (a molecule increased dramatically in diabetic sEV) mimic and blocked by a miRb-130b-3p inhibitor. Molecular studies identified PGC-1α (i.e. PGC-1α1/-a) as the direct downstream target of miR-130b-3p, whose downregulation causes mitochondrial dysfunction and apoptosis. Finally, treatment with diabetic adipocyte-derived sEV or a miR-130b-3p mimic significantly enhanced mitochondrial reactive oxygen species (ROS) production in SI/R cardiomyocytes. Conversely, treatment with a miR-130b-3p inhibitor or overexpression of PGC-1α extremely attenuated diabetic sEV-induced ROS production.
Conclusion: We obtained the first evidence that diabetic sEV promotes oxidative stress and mitochondrial-mediated cardiomyocyte apoptotic cell death, exacerbating MI/R injury. These pathological phenotypes were mediated by miR-130b-3p-induced suppression of PGC-1α expression and subsequent mitochondrial ROS production. Targeting miR-130b-3p mediated cardiomyocyte apoptosis may be a novel strategy for attenuating diabetic exacerbation of MI/R injury.
Keywords: Apoptosis; Diabetes; Extracellular vesicle; Myocardial ischemia/reperfusion injury.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Oikonomou E.K., Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nat. Rev. Cardiol. 2018;16:83–99. - PubMed
-
- Duckworth W., Abraira C., Moritz T., Reda D., Emanuele N., Reaven P.D., Zieve F.J., Marks J., Davis S.N., Hayward R., Warren S.R., Goldman S., McCarren M., Vitek M.E., Henderson W.G., Huang G.D. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 2009;360:129–139. - PubMed
-
- Das Pradhan A., Glynn R.J., Fruchart J.C., MacFadyen J.G., Zaharris E.S., Everett B.M., Campbell S.E., Oshima R., Amarenco P., Blom D.J., Brinton E.A., Eckel R.H., Elam M.B., Felicio J.S., Ginsberg H.N., Goudev A., Ishibashi S., Joseph J., Kodama T., Koenig W., Leiter L.A., Lorenzatti A.J., Mankovsky B., Marx N., Nordestgaard B.G., Pall D., Ray K.K., Santos R.D., Soran H., Susekov A., Tendera M., Yokote K., Paynter N.P., Buring J.E., Libby P., Ridker P.M., Investigators P. Triglyceride lowering with pemafibrate to reduce cardiovascular risk. N. Engl. J. Med. 2022;387:1923–1934. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

