Prevention of Stroke in Intracerebral Haemorrhage Survivors with Atrial Fibrillation: Rationale and Design for PRESTIGE-AF Trial
- PMID: 39740761
- PMCID: PMC11961226
- DOI: 10.1055/a-2496-5492
Prevention of Stroke in Intracerebral Haemorrhage Survivors with Atrial Fibrillation: Rationale and Design for PRESTIGE-AF Trial
Abstract
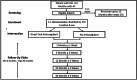
Adequate secondary prevention in survivors of intracerebral hemorrhage (ICH) who also have atrial fibrillation (AF) is a long-standing clinical dilemma because these patients are at increased risk of recurrent ICH as well as of ischemic stroke. The efficacy and safety of oral anticoagulation, the standard preventive medication for ischemic stroke patients with AF, in ICH patients with AF are uncertain. PRESTIGE-AF is an international, phase 3b, multi-center, randomized, open, blinded end-point assessment (PROBE) clinical trial that compared the efficacy and safety of direct oral anticoagulants (DOACs) with no DOAC (either no antithrombotic treatment or any antiplatelet drug). Randomization occurred in a 1:1 ratio and stratification was based on ICH location and sex. The two co-primary binary endpoints included ischemic stroke and recurrent ICH which will be analyzed hierarchically according to the intention-to-treat principle. Secondary efficacy endpoints encompassed all-stroke and systemic embolism, all-cause and cardiovascular mortality, major adverse cardiac events, and net clinical benefit. Secondary safety endpoints included any major hemorrhage and intracranial hemorrhage. All outcome events were adjudicated by an independent committee. Results of PRESTIGE-AF are expected to support risk-adjusted secondary prevention in ICH survivors with AF and to inform clinical guideline recommendations.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/).
Conflict of interest statement
All the authors are a part of European Union's Horizon 2020 research and innovation program under grant agreement No. 754517 (PRESTIGE-AF). E.K. received Speaker Honoraria/Advisory boards/Travel grants from Amgen, AstraZeneca, Bayer, Elpen, Innovis, Pfizer, Sanofi. All outside the submitted work. P.H. received Institutional Research Grants by German Ministry of Research and Education, German Research Foundation, Federal Joint Committee (G-BA) within the Innovation fond, German Cancer Aid, German Heart Foundation, Bavarian State, Robert Koch Institute, University Hospital Heidelberg. Y.W. received support from The National Institute for Health and Care Research (NIHR) under its Program Grants for Applied Research (NIHR202339). P.B.N. received Institutional support by grants of Daiichi Sankyo; Consultancy: Daiichi-Sankoy, Bayer; Speaker Honoraria/Advisory boards: SERVIER, Baye. V.C. received consultancy fees from Bayer Steering Committee Oceanic AF; Speakers: Bayer, BMS-Pfizer alliance and Daiichi-Sankyo. Leadership Role: WSO Treasurer. D.L. received institutional support for investigator-initiated quality improvement grants: Bristol-Myers Squibb and Pfizer; Speaker: Bayer, Boehringer Ingelheim, and Bristol-Myers Squibb/Pfizer; Consultancy: Bristol-Myers Squibb and Boehringer Ingelheim; all outside the submitted work. P.R. received institution support by: Boehringer Ingelheim, Bayer; Speaker: Boehringer Ingelheim, Bayer, Pfizer. Participation on Data Safety: CLOSURE-AF (Institution) NISCI (no payment). W.H. received institutional support by grants from Boehringer Ingelheim and Daiichi-Sankyo. R. V. received speaker Honoraria/Advisory boards: AstraZeneca, Bayer, BMS-Pfizer alliance. Research support: Bayer, BMS-Pfizer alliance, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, Biogen. All other authors declare no conflict of interest.
Figures
References
-
- European Registers of Stroke (EROS) Investigators . Heuschmann P U, Di Carlo A, Bejot Y et al. Incidence of stroke in Europe at the beginning of the 21st century. Stroke. 2009;40(05):1557–1563. - PubMed
-
- Global Burden of Diseases, Injuries, Risk Factors Study 2010 (GBD 2010) ; GBD Stroke Experts Group . Krishnamurthi R V, Feigin V L, Forouzanfar M H et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1(05):e259–e281. - PMC - PubMed
-
- GBD 2021 Stroke Risk Factor Collaborators . Global, regional, and national burden of stroke and its risk factors, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024;23(10):973–1003. - PubMed
-
- Flaherty M L, Kissela B, Woo D et al. The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology. 2007;68(02):116–121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical