Anamorelin and Conduction Defects: A Literature Review and Analysis of the Japanese Pharmacovigilance Database
- PMID: 39740881
- PMCID: PMC11705112
- DOI: 10.21873/invivo.13842
Anamorelin and Conduction Defects: A Literature Review and Analysis of the Japanese Pharmacovigilance Database
Abstract
Background/aim: Cancer cachexia is characterized by weight loss with a specific decrease in skeletal muscle and adipose tissue. In Japan, anamorelin, which has a novel mechanism of action, was approved in 2021 for the treatment of cancer cachexia. However, little information is available on its safety in routine clinical care, in particular the occurrence of conduction defects as adverse reactions. Therefore, this study evaluated the risk and time to onset of anamorelin-related conduction defects by performing a literature review and evaluating the Japanese pharmacovigilance database.
Patients and methods: We reviewed the literature from April 2000 to June 2024 to identify reports of anamorelin-related conduction defects and analyzed data from April 2004 to December 2023 in the Japanese Adverse Drug Event Report (JADER) database. Using the database, we calculated reporting odds ratios (RORs) with 95% confidence intervals (CIs) and adjusted RORs (95%CIs) by considering whether patients were taking concomitant medications that can cause QT prolongation. In addition, we investigated outcomes and time to onset.
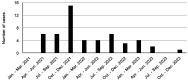
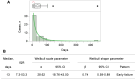
Results: The literature review identified seven cases of conduction defects. All cases occurred within approximately three weeks after starting treatment, and all patients recovered. The JADER database contained 537 cases of adverse reactions to anamorelin. The adjusted ROR (95%CI) of conduction defects was 20.00 (14.86-26.91), and the median time to onset was 13 days. Poor clinical outcomes occurred in only a few cases.
Conclusion: Performing frequent cardiac electrograms for two to three weeks after starting anamorelin may help to quickly identify anamorelin-related conduction defects.
Keywords: Anamorelin; Japanese Adverse Drug Event Report database; adverse reactions; conduction defect; review; time to onset.
Copyright © 2025, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors have no conflicts of interest to disclose in relation to this study.
Figures

Similar articles
-
Immune Checkpoint Inhibitor-induced Uveitis: Disproportionality and Timing Analyses of the Japanese Pharmacovigilance Database.Anticancer Res. 2025 May;45(5):2215-2223. doi: 10.21873/anticanres.17595. Anticancer Res. 2025. PMID: 40295055
-
Real-world safety and effectiveness of anamorelin for cancer cachexia: Interim analysis of post-marketing surveillance in Japan.Cancer Med. 2024 May;13(9):e7170. doi: 10.1002/cam4.7170. Cancer Med. 2024. PMID: 38693813 Free PMC article.
-
Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials.Lancet Oncol. 2015 Jan;16(1):108-16. doi: 10.1016/S1470-2045(14)71154-4. Epub 2014 Dec 16. Lancet Oncol. 2015. PMID: 25524795
-
Anamorelin in Japanese patients with cancer cachexia: an update.Curr Opin Support Palliat Care. 2023 Sep 1;17(3):162-167. doi: 10.1097/SPC.0000000000000658. Epub 2023 Jun 30. Curr Opin Support Palliat Care. 2023. PMID: 37389636 Review.
-
Anamorelin for cancer cachexia.Drugs Today (Barc). 2022 Mar;58(3):97-104. doi: 10.1358/dot.2022.58.3.3381585. Drugs Today (Barc). 2022. PMID: 35274629 Review.
References
-
- Arends J, Strasser F, Gonella S, Solheim TS, Madeddu C, Ravasco P, Buonaccorso L, de van der Schueren MAE, Baldwin C, Chasen M, Ripamonti CI, ESMO Guidelines Committee Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines(✩) ESMO Open. 2021;6(3):100092. doi: 10.1016/j.esmoop.2021.100092. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
