In Vivo Antitumor Activity of the PD-1/PD-L1 Inhibitor SCL-1 in Various Mouse Tumor Models
- PMID: 39740910
- PMCID: PMC11705142
- DOI: 10.21873/invivo.13805
In Vivo Antitumor Activity of the PD-1/PD-L1 Inhibitor SCL-1 in Various Mouse Tumor Models
Abstract
Background/aim: Immune checkpoint blockade has achieved great success as a targeted immunotherapy for solid cancers. However, small molecules that inhibit programmed death 1/programmed death ligand 1 (PD-1/PD-L1) binding are still being developed and have several advantages, such as high bioavailability. Previously, we reported a novel PD-1/PD-L1-inhibiting small compound, SCL-1, which showed potent antitumor effects on PD-L1+ tumors. These effects were dependent on CD8+ T-cell infiltration and PD-L1 expression on tumors. The present study investigated the in vivo antitumor activity of SCL-1 in various mouse syngeneic tumor models.
Materials and methods: Twelve syngeneic mice models of tumors, such as colon, breast, bladder, kidney, pancreatic, non-small cell lung cancers, melanoma, and lymphomas, were used for in vivo experiments. Tumor mutation burden (TMB) was analyzed by whole exome sequencing (WES) using reference DNA from mouse blood. The proportion of CD8+ T-cells infiltrating tumors before and after treatment was assessed using flow cytometry and immunohistochemistry (IHC).
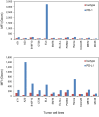
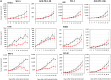
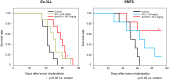
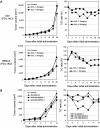
Results: SCL-1 had a markedly greater antitumor effect (11 sensitive tumors and 1 resistant tumor among the 12 tumor types) than the anti-mouse PD-1 antibody (8 sensitive tumors and 4 resistant tumors). In addition, the tumor growth inhibition rate (%) was more closely associated with TMB in the SCL-1 group than in the anti-PD-1 antibody group. Furthermore, in vivo experiments using PD-L1 gene knockout and lymphocyte-depletion technologies demonstrated that the antitumor activity of SCL-1 was dependent on CD8+ T-cell infiltration and PD-L1 expression in tumors.
Conclusion: SCL-1 has great potential as an oral immunotherapy that targets immune checkpoint molecules in cancer treatment.
Keywords: PD-1/PD-L1 inhibitor; mouse syngeneic tumors; small chemical compound; tumor mutation burden (TMB); tumor-infiltrating lymphocyte (TIL).
Copyright © 2025, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors declare that they have no conflicts of interest in relation to this study.
Figures


References
-
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. - DOI - PMC - PubMed
-
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. - DOI - PMC - PubMed
-
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. - DOI - PMC - PubMed
-
- Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA, National Comprehensive Cancer Network Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–1768. doi: 10.1200/JCO.2017.77.6385. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
