Low-dose Imatinib Efficacy in a Gastrointestinal Stromal Tumor Patient With KIT Exon 11 W557_K558 Deletion
- PMID: 39740915
- PMCID: PMC11705098
- DOI: 10.21873/invivo.13857
Low-dose Imatinib Efficacy in a Gastrointestinal Stromal Tumor Patient With KIT Exon 11 W557_K558 Deletion
Abstract
Background/aim: Gastrointestinal stromal tumors (GISTs) are rare cancers originating from Cajal's stromal cells in the gastrointestinal tract. The most common driver mutation in these cancers is the KIT mutation. This report presents a case of response to low-dose imatinib in a patient with GIST harboring KIT exon 11 W557_K558 deletion.
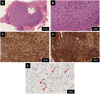
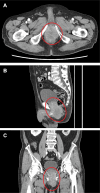
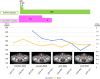
Case report: In June 2023, an 82-year-old male developed perineal pain. Computed tomography (CT) imaging revealed a mass measuring >9 cm, extending from the rectum to the prostate. Submucosal tumor biopsy revealed a tumor with CD117-positive and DOG1-positive spindle-shaped cells leading to a diagnosis of GIST. c-Kit gene mutation analysis detected a W557_K558 deletion in exon 11. Treatment with imatinib (400 mg/day) was initiated in late October 2023 to preserve organ function; the dose was reduced to 300 mg/day after 5 days of treatment and further reduced to 200 mg/day after 10 days, with continued treatment at this dose. The CT performed in January, April, and June 2024 showed that the rectal GIST had shrunk. The blood imatinib concentration remained at approximately 650 ng/ml from January to March 2024 and decreased to 391 ng/ml in May 2024.
Conclusion: The response rate of GISTs to imatinib is influenced by genetic mutations. GIST with KIT exon 11 W557_K558 deletion is associated with a high risk of recurrence. In vitro data showed that low-dose imatinib was effective in such cases. Low-dose imatinib is a treatment option for patients with GIST harboring KIT exon 11 W557_K558 deletion who are intolerant to high-dose imatinib.
Keywords: Gastrointestinal stromal tumor; exon 11 W557_K558 deletion; imatinib.
Copyright © 2025, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
KM has received research grants from Daiichi Sankyo, MSD, Eli Lilly, and Gilead Sciences (to institution); honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from MSD, Kyowa Kirin, Eli Lilly, Daiichi Sankyo, Eisai, and Chugai; advisory board fees from Gilead Sciences, Daiichi Sankyo, Pfizer, Genmab, Takeda, Eisai, and Nippon Shinyaku.
Figures
Similar articles
-
Oncogene mutational analysis in imatinib naive population of gastrointestinal stromal tumor patients.Cell Mol Biol (Noisy-le-grand). 2020 Dec 31;66(8):26-32. Cell Mol Biol (Noisy-le-grand). 2020. PMID: 34174973
-
Effect of KIT and PDGFRA Mutations on Survival in Patients With Gastrointestinal Stromal Tumors Treated With Adjuvant Imatinib: An Exploratory Analysis of a Randomized Clinical Trial.JAMA Oncol. 2017 May 1;3(5):602-609. doi: 10.1001/jamaoncol.2016.5751. JAMA Oncol. 2017. PMID: 28334365 Free PMC article. Clinical Trial.
-
Remarkable effects of imatinib in a family with young onset gastrointestinal stromal tumors and cutaneous hyperpigmentation associated with a germline KIT-Trp557Arg mutation: case report and literature overview.Fam Cancer. 2018 Apr;17(2):247-253. doi: 10.1007/s10689-017-0024-8. Fam Cancer. 2018. PMID: 28710566 Review.
-
The INSIGHT study: a randomized, Phase III study of ripretinib versus sunitinib for advanced gastrointestinal stromal tumor with KIT exon 11 + 17/18 mutations.Future Oncol. 2024;20(27):1973-1982. doi: 10.1080/14796694.2024.2376521. Epub 2024 Sep 4. Future Oncol. 2024. PMID: 39229786 Free PMC article.
-
KIT Exon 9-Mutated Gastrointestinal Stromal Tumours: Biology and Treatment.Chemotherapy. 2022;67(2):81-90. doi: 10.1159/000521751. Epub 2022 Jan 4. Chemotherapy. 2022. PMID: 34983047 Review.
References
-
- de Pinieux G, Karanian M, Le Loarer F, Le Guellec S, Chabaud S, Terrier P, Bouvier C, Batistella M, Neuville A, Robin YM, Emile JF, Moreau A, Larousserie F, Leroux A, Stock N, Lae M, Collin F, Weinbreck N, Aubert S, Mishellany F, Charon-Barra C, Croce S, Doucet L, Quintin-Rouet I, Chateau MC, Bazille C, Valo I, Chetaille B, Ortonne N, Brouchet A, Rochaix P, Demuret A, Ghnassia JP, Mescam L, Macagno N, Birtwisle-Peyrottes I, Delfour C, Angot E, Pommepuy I, Ranchere D, Chemin-Airiau C, Jean-Denis M, Fayet Y, Courrèges JB, Mesli N, Berchoud J, Toulmonde M, Italiano A, Le Cesne A, Penel N, Ducimetiere F, Gouin F, Coindre JM, Blay JY, NetSarc/RePPS/ResSos and French Sarcoma Group- Groupe d’Etude des Tumeurs Osseuses (GSF-GETO) networks Nationwide incidence of sarcomas and connective tissue tumors of intermediate malignancy over four years using an expert pathology review network. PLoS One. 2021;16(2):e0246958. doi: 10.1371/journal.pone.0246958. - DOI - PMC - PubMed
-
- Wozniak A, Rutkowski P, Piskorz A, Ciwoniuk M, Osuch C, Bylina E, Sygut J, Chosia M, Rys J, Urbanczyk K, Kruszewski W, Sowa P, Siedlecki J, Debiec-Rychter M, Limon J. Prognostic value of KIT/PDGFRA mutations in gastrointestinal stromal tumours (GIST): Polish Clinical GIST Registry experience. Ann Oncol. 2012;23(2):353–360. doi: 10.1093/annonc/mdr127. - DOI - PubMed
-
- Heinrich MC, Owzar K, Corless CL, Hollis D, Borden EC, Fletcher CD, Ryan CW, von Mehren M, Blanke CD, Rankin C, Benjamin RS, Bramwell VH, Demetri GD, Bertagnolli MM, Fletcher JA. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008;26(33):5360–5367. doi: 10.1200/JCO.2008.17.4284. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
