Safety and Efficacy of Imeglimin for Type 2 Diabetes Mellitus in Patients With Heart Failure
- PMID: 39740918
- PMCID: PMC11705109
- DOI: 10.21873/invivo.13838
Safety and Efficacy of Imeglimin for Type 2 Diabetes Mellitus in Patients With Heart Failure
Abstract
Background/aim: Imeglimin, a novel oral antidiabetic agent, was approved in 2021 for the treatment of type 2 diabetes mellitus (T2DM). Phase III clinical trials demonstrated its safety and efficacy in managing T2DM. However, its safety profile in patients with heart failure has not been thoroughly evaluated in real-world clinical settings.
Patients and methods: We analyzed cases of patients with heart failure (stage B or higher) who were newly prescribed imeglimin, based on electronic medical records from June 2022 to June 2024. Baseline clinical data at the initiation of imeglimin therapy were collected, and cardiovascular events, adverse effects (e.g., lactic acidosis), and blood test results, including glycated hemoglobin A1c (HbA1c), were assessed as of July 2024.
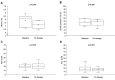
Results: A total of 21 patients met the inclusion criteria. HbA1c levels significantly decreased after an average of 312.1±205.8 days of imeglimin therapy (baseline vs. on therapy: 8.2±1.0% vs. 7.5±0.7%, p=0.001). Alanine aminotransferase levels were also significantly reduced (baseline vs. on therapy: 30.9±23.8 IU/l vs. 22.0±12.3 IU/l, p=0.022). No adverse drug reactions were observed during the treatment period. Major adverse cardiovascular events occurred in three patients (14%), although a clear association with imeglimin remains uncertain.
Conclusion: Imeglimin demonstrated safety and efficacy in T2DM in patients with coexisting heart failure.
Keywords: ALT; AST; HbA1c; Imeglimin; heart failure; lactic acidosis; type 2 diabetes.
Copyright © 2025, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
AH received a lecture fee from Sumitomo Pharma Co., Ltd.
Figures


References
-
- Shi K, Zhang G, Fu H, Li XM, Gao Y, Shi R, Xu HY, Li Y, Guo YK, Yang ZG. Glycemic control and clinical outcomes in diabetic patients with heart failure and reduced ejection fraction: insight from ventricular remodeling using cardiac MRI. Cardiovasc Diabetol. 2024;23(1):148. doi: 10.1186/s12933-024-02243-w. - DOI - PMC - PubMed
-
- Kitakata H, Endo J, Hashimoto S, Mizuno E, Moriyama H, Shirakawa K, Goto S, Katsumata Y, Fukuda K, Sano M. Imeglimin prevents heart failure with preserved ejection fraction by recovering the impaired unfolded protein response in mice subjected to cardiometabolic stress. Biochem Biophys Res Commun. 2021;572:185–190. doi: 10.1016/j.bbrc.2021.07.090. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
