Association Between Hypnotics, Accidents, and Injuries: A Study Based on the Adverse Drug Event Reporting Database in Japan
- PMID: 39740920
- PMCID: PMC11705136
- DOI: 10.21873/invivo.13846
Association Between Hypnotics, Accidents, and Injuries: A Study Based on the Adverse Drug Event Reporting Database in Japan
Abstract
Background/aim: The use of hypnotic drugs can lead to accidents and injuries. However, few reports have shown their association with these events after adjusting for many concomitant medications. This study aimed to determine whether the use of hypnotic drugs was associated with accidents and injuries.
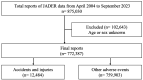
Patients and methods: Using the Japanese Adverse Event Reporting Database, 772,387 reports published between September 2023 and April 2004 were analyzed. Reporting odds ratios (RORs) and 95% confidence intervals (CIs) for accidents and injuries associated with each hypnotic drug were calculated after adjusting for potential confounders.
Results: Of the total, 12,484 reports indicated association of hypnotic drugs with accidents and injuries. The use of each hypnotic drug was associated with accidents, injuries, and other adverse events. However, a multivariate analysis adjusted for age, sex, reporting year, and concomitant medications showed a considerable decrease in ROR for melatonin receptor agonists (adjusted ROR=1.26; 95%CI=1.03-1.55) and dual orexin receptor antagonists (DORAs) (adjusted ROR=1.04; 95%CI=0.86-1.25). Particularly in DORAs, a loss of signal for accidents and injuries was observed.
Conclusion: The risk of accidents and injuries may vary with hypnotic drug use; however, DORAs may be less frequently associated with these events. The results of this study provide useful information for the selection of hypnotic drugs.
Keywords: Accident; adverse drug event; hypnotics; injury.
Copyright © 2025, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors have no conflicts of interest to declare in relation to this study.
References
-
- Khong E, Sim MG, Hulse G. Benzodiazepine dependence. Aust Fam Physician. 2004;33(11):923–926. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous