Kappa opioid receptor internalisation-induced p38 nuclear translocation suppresses glioma progression
- PMID: 39741108
- PMCID: PMC11867070
- DOI: 10.1016/j.bja.2024.09.031
Kappa opioid receptor internalisation-induced p38 nuclear translocation suppresses glioma progression
Abstract
Background: Recent studies have implicated a role for perioperative medications in determining patient outcomes after surgery for malignant tumours, including relapse and metastasis.
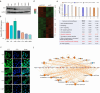
Methods: A combined approach spanned molecular, cellular, and organismal levels, including bioinformatics, immunohistochemical staining of clinical and animal samples, RNA sequencing of glioblastoma multiforme (GBM) cells with Ingenuity Pathway Analysis, lentiviral-mediated gene expression modulation, in vitro cell experiments, and in vivo orthotopic tumour transplantation.
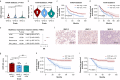
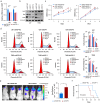
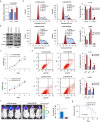
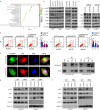
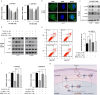
Results: We observed a significant correlation between increased kappa opioid receptor (KOP receptor) expression and better prognosis in patients with glioma. Exogenous KOP receptor overexpression in GBM cells in vitro induced cell cycle arrest, suppressed cell growth, and promoted apoptosis. Conversely, reducing KOP receptor expression in GBM cells reduced the proportion of cells in S and G2/M phases, accelerating cell growth. KOP receptor overexpression inhibited glioma cell growth and prolonged survival in mice in vivo, while KOP receptor knockdown had the opposite effect. Mechanistically, internalised KOP receptors were found to bind cytoplasmic p38, facilitating its nuclear translocation and phosphorylation, which influences downstream gene expression. The selective KOP receptor agonist TRK-820 triggered KOP receptor internalisation, activated the p38 pathway, and diminished glioma cell viability in vitro.
Conclusions: This combined molecular, cellular, and in vivo approach supports use of KOP receptor agonists as potential adjuvant therapeutics for glioma.
Keywords: KOP agonist; KOP receptor; glioblastoma multiforme; p38 MAP kinase; receptor internalisation.
Copyright © 2024 British Journal of Anaesthesia. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interest The authors declare that they have no conflicts of interest.
Figures
References
-
- Nicholson J.G., Fine H.A. Diffuse glioma heterogeneity and its therapeutic implications. Cancer Discov. 2021;11:575–590. - PubMed
-
- Tan A.C., Ashley D.M., López G.Y., Malinzak M., Friedman H.S., Khasraw M. Management of glioblastoma: state of the art and future directions. CA Cancer J Clin. 2020;70:299–312. - PubMed
-
- Hiller J.G., Perry N.J., Poulogiannis G., Riedel B., Sloan E.K. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol. 2018;15:205–218. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

