Osimertinib for EGFR-Mutant NSCLC Patients With Acquired T790M and EGFR Amplification After First-Generation EGFR-TKI Resistance
- PMID: 39741120
- PMCID: PMC11875782
- DOI: 10.1111/cas.16437
Osimertinib for EGFR-Mutant NSCLC Patients With Acquired T790M and EGFR Amplification After First-Generation EGFR-TKI Resistance
Abstract
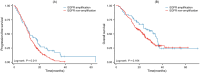
Third-generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) is the standard therapy for patients harboring T790M after first-generation EGFR-TKI resistance. However, the impact of acquired EGFR amplification on the efficacy of third-generation EGFR-TKI against T790M remains uncertain. We aimed to investigate whether the presence of acquired EGFR amplification after first-generation EGFR-TKI resistance influences the efficacy of third-generation EGFR-TKI in patients with advanced non-small-cell lung cancer (NSCLC). We reviewed data from 275 advanced NSCLC patients harboring T790M after first-generation EGFR-TKI resistance. Patients were categorized into two groups based on the presence or absence of acquired EGFR amplification identified through next-generation sequencing (NGS) after first-line EGFR-TKI treatment. We evaluated the efficacy of osimertinib used as a second-line treatment. Among these patients, 59 exhibited acquired EGFR amplification, while 216 did not. The median progression-free survival (PFS) was 12.20 months in the EGFR amplification group and 12.03 months in the non-amplification group (p = 0.011), with median overall survival (OS) of 33.90 months and 23.30 months, respectively (p = 0.164). Multivariate analysis of PFS revealed that acquired EGFR amplification and EGFR 19del were independent prognostic factors for patients with T790M undergoing osimertinib. Additionally, subgroup analysis indicated a prolonged PFS in patients with EGFR 19del compared to those with EGFR 21L858R (p = 0.034) in the EGFR amplification group. Following first-generation EGFR-TKI resistance, advanced EGFR-mutant NSCLC patients harboring both acquired T790M and EGFR amplification are likely to experience enhanced PFS with osimertinib. This phenomenon is particularly noteworthy among individuals with EGFR 19del.
Keywords: EGFR; EGFR amplification; EGFR‐TKI; NSCLC; T790M.
© 2024 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Osimertinib alone as second-line treatment for brain metastases (BM) control may be more limited than for non-BM in advanced NSCLC patients with an acquired EGFR T790M mutation.Respir Res. 2021 May 11;22(1):145. doi: 10.1186/s12931-021-01741-9. Respir Res. 2021. PMID: 33975616 Free PMC article.
-
Analysis of genomic alternations in epidermal growth factor receptor (EGFR)-T790M-mutated non-small cell lung cancer (NSCLC) patients with acquired resistance to osimertinib therapy.Clin Transl Oncol. 2025 May;27(5):1967-1979. doi: 10.1007/s12094-024-03727-7. Epub 2024 Sep 24. Clin Transl Oncol. 2025. PMID: 39317868 Free PMC article.
-
Audit of Molecular Mechanisms of Primary and Secondary Resistance to Various Generations of Tyrosine Kinase Inhibitors in Known Epidermal Growth Factor Receptor-Mutant Non-small Cell Lung Cancer Patients in a Tertiary Centre.Clin Oncol (R Coll Radiol). 2022 Nov;34(11):e451-e462. doi: 10.1016/j.clon.2022.06.003. Epub 2022 Jul 7. Clin Oncol (R Coll Radiol). 2022. PMID: 35810049
-
Efficacy and safety of osimertinib in treating EGFR-mutated advanced NSCLC: A meta-analysis.Int J Cancer. 2019 Jul 1;145(1):284-294. doi: 10.1002/ijc.32097. Epub 2019 Jan 20. Int J Cancer. 2019. PMID: 30613959 Free PMC article.
-
Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients.Ann Oncol. 2018 Jan 1;29(suppl_1):i20-i27. doi: 10.1093/annonc/mdx704. Ann Oncol. 2018. PMID: 29462255 Review.
Cited by
-
EGFR mutations in non-small cell lung cancer: Classification, characteristics and resistance to third-generation EGFR-tyrosine kinase inhibitors (Review).Oncol Lett. 2025 Jun 2;30(2):375. doi: 10.3892/ol.2025.15121. eCollection 2025 Aug. Oncol Lett. 2025. PMID: 40503037 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

