Trogocytosis-mediated immune evasion in the tumor microenvironment
- PMID: 39741180
- PMCID: PMC11799389
- DOI: 10.1038/s12276-024-01364-2
Trogocytosis-mediated immune evasion in the tumor microenvironment
Abstract
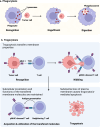
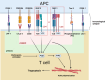
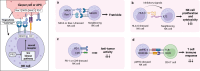
Trogocytosis is a dynamic cellular process characterized by the exchange of the plasma membrane and associated cytosol during cell-to-cell interactions. Unlike phagocytosis, this transfer maintains the surface localization of transferred membrane molecules. For example, CD4 T cells engaging with antigen-presenting cells undergo trogocytosis, which facilitates the transfer of antigen-loaded major histocompatibility complex (MHC) class II molecules from antigen-presenting cells to CD4 T cells. This transfer results in the formation of antigen-loaded MHC class II molecule-dressed CD4 T cells. These "dressed" CD4 T cells subsequently participate in antigen presentation to other CD4 T cells. Additionally, trogocytosis enables the acquisition of immune-regulatory molecules, such as CTLA-4 and Tim3, in recipient cells, thereby modulating their anti-tumor immunity. Concurrently, donor cells undergo plasma membrane loss, and substantial loss can trigger trogocytosis-mediated cell death, termed trogoptosis. This review aims to explore the trogocytosis-mediated transfer of immune regulatory molecules and their implications within the tumor microenvironment to elucidate the underlying mechanisms of immune evasion in cancers.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Hudson, L., Sprent, J., Miller, J. F. A. P. & Playfair, J. H. L. B cell-derived immunoglobulin on activated mouse T lymphocytes. Nature251, 60–62 (1974). - PubMed
-
- Huang, J.-F. et al. TCR-mediated internalization of peptide-MHC complexes acquired by T cells. Science286, 952–954 (1999). - PubMed
-
- Joly, E. & Hudrisier, D. What is trogocytosis and what is its purpose? Nat. Immunol.4, 815–815 (2003). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

