Modulation of pain sensitivity by Ascl1- and Lhx6-dependent GABAergic neuronal function in streptozotocin diabetic mice
- PMID: 39741412
- PMCID: PMC11852955
- DOI: 10.1016/j.ymthe.2024.12.039
Modulation of pain sensitivity by Ascl1- and Lhx6-dependent GABAergic neuronal function in streptozotocin diabetic mice
Abstract
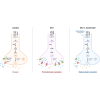
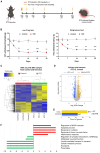
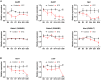
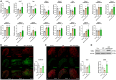
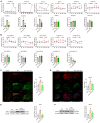
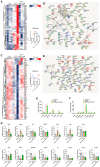
Painful diabetic neuropathy commonly affects the peripheral nervous system in individuals with diabetes. However, the pathological processes and mechanisms underlying diabetic neuropathic pain remain unclear. We aimed to identify the overall profiles and screen for genes potentially involved in pain mechanisms using transcriptome analysis of the dorsal root ganglion of diabetic mice treated with streptozotocin (STZ). Using RNA sequencing, we identified differentially expressed genes between streptozotocin-treated diabetic mice and controls, focusing on altered GABAergic neuron-related genes and inflammatory pathways. Behavioral and molecular analyses revealed a marked reduction in GABAergic neuronal markers (GAD65, GAD67, VGAT) and increased pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) in the diabetic group compared with controls. Intrathecal administration of lentiviral vectors expressing transcription factors Ascl1 and Lhx6 reversed pain hypersensitivity and restored normal expression of GABAergic genes and inflammatory mediators. Protein-protein interaction network analysis revealed five key proteins influenced by Ascl1 and Lhx6 treatment, including those in the JunD/FosB/C-fos signaling pathway. These findings suggest that Ascl1 and Lhx6 mitigate diabetic neuropathic pain by modulating GABAergic neuronal function, pro-inflammatory responses, and pain-related channels (TRPV1, Nav1.7). These results provide a basis for developing transcription factor-based therapies targeting GABAergic neurons for diabetic neuropathic pain relief.
Keywords: GABAergic neuron; RNA sequencing; diabetic neuropathic pain; dorsal root ganglion; transcription factor.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

