Selective deletion of Tsc1 from mouse cerebellar Purkinje neurons drives sex-specific behavioral impairments linked to autism
- PMID: 39741564
- PMCID: PMC11685134
- DOI: 10.3389/fnbeh.2024.1474066
Selective deletion of Tsc1 from mouse cerebellar Purkinje neurons drives sex-specific behavioral impairments linked to autism
Abstract
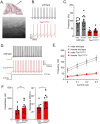
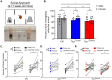
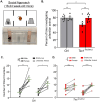
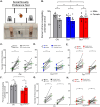
There is a striking sex bias in the prevalence and severity of autism spectrum disorder (ASD) with 80% of diagnoses occurring in males. Because the molecular etiology of ASD is likely combinatorial, including interactions across multiple genetic and environmental factors, it is difficult to investigate the physiological mechanisms driving sex-specific differences. Loss of function mutations in TSC1 result in dysregulated mTORC1 signaling and underlie a multi-system disorder known as tuberous sclerosis (TSC). Interestingly, more than 50% of individuals diagnosed with TSC are also diagnosed with ASD, making TSC mutations one of the most prevalent monogenic causes of ASD. Mice harboring targeted deletion of Tsc1 selectively in cerebellar Purkinje neurons, referred to here as Tsc1mut/mut , have multiple ASD-linked behavioral impairments, including deficits in social interactions, motor coordination, and vocalizations. However, these ASD-linked behavioral deficits have only been investigated using male Tsc1mut/mut animals. Here, we used cohorts of male and female Tsc1mut/mut animals to determine if behavioral impairments, previously identified in this model, are similar across sex. Specifically, we measured balance and motor coordination and social interaction behaviors in two age groups across sex. We determined balance and motor coordination deficits are similar in male and female Tsc1mut/mut mice, and that deficits in the firing of Tsc1mut/mut Purkinje neurons located in the cerebellar vermis are also similar across sex. However, impairments in social approach behavior were found to be significantly more severe in Tsc1mut/mut males compared to females. These results indicate the selective deletion of Tsc1 in Purkinje neurons differentially impairs cerebellar circuits based on sex.
Keywords: TSC; cerebellum; motor coordination; sexual dimorphism; social interaction; tuberous sclerosis.
Copyright © 2024 Lawson, Lipovsek, Brown, Jena, Osko and Ransdell.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Update of
-
Selective deletion of Tsc1 from mouse cerebellar Purkinje neurons drives sex-specific behavioral impairments linked to autism.bioRxiv [Preprint]. 2024 Aug 7:2024.08.07.607071. doi: 10.1101/2024.08.07.607071. bioRxiv. 2024. Update in: Front Behav Neurosci. 2024 Dec 17;18:1474066. doi: 10.3389/fnbeh.2024.1474066. PMID: 39211158 Free PMC article. Updated. Preprint.
References
-
- Au K. S., Williams A. T., Steve Roach E., Batchelor L., Sparagana S. P., Delgado M. R., et al. . (2007). Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet. Med. 9, 88–100. doi: 10.1097/GIM.0b013e31803068c7, PMID: - DOI - PubMed
-
- Barski J. J., Dethleffsen K., Meyer M. (2000). Cre recombinase expression in cerebellar purkinje cells. Genesis 28, 93–98. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

