Air Storage Impact on Surface Evolution of Stoichiometric and Li-Rich NMC811
- PMID: 39741816
- PMCID: PMC11683608
- DOI: 10.1021/acsomega.4c06636
Air Storage Impact on Surface Evolution of Stoichiometric and Li-Rich NMC811
Abstract
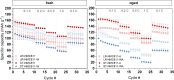
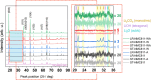
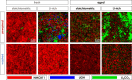
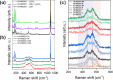
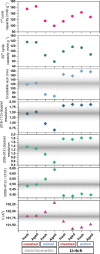
In recent years, a type of layered oxide, LiNi x Mn y Co z O2 (NMC) where x+y+z = 1, has become the preferred cathode material for electric vehicle (EV) batteries. Despite some disorder in the crystal structure due to Li+/Ni2+ cation mixing, the composition offers a high specific capacity of up to 200 mAh g-1 at 4.3 V vs Li|Li+. The objective of this study is to comprehensively evaluate the structural and electrochemical changes in NMC811 after storage in ambient conditions. In this report, we study stoichiometric and Li-rich NMC811 in terms of their structural, morphological, and electrochemical differences. Following literature reports, a rigorous aqueous washing procedure was used alternatively to remove a possible lithium excess from the NMC surface. The findings of this study hold immense significance as they focus on the potential challenges that may arise due to the remaining lithium content or Li+ extraction from the near-surface NMC811 materials. There is no consensus in the literature on whether excess lithium can harm the material's structural and electrochemical properties, reduce performance and safety concerns, or be beneficial regarding its protective properties, for Ni-rich NMC. Proper treatment of as-synthesized Ni-rich NMCs helps to develop procedures to address the residual lithium compounds issues, leading to enhanced performance and safety. Here with this report, we show another aspect not being considered in the literature before, regarding morphological NMC811 reshaping and a mechanism of LRC transition and growth due to aging. In addition, we linked the selected structural parameters to the electrochemical performance of various NMC811 materials. We discuss the well-known structural factors and their limitations and introduce a doublet resolution criterion that can help in predicting electrochemical performance.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Li J.; Downie L. E.; Ma L.; Qiu W.; Dahn J. R. Study of the Failure Mechanisms of LiNi 0.8 Mn 0.1 Co 0.1 O 2 Cathode Material for Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162 (7), A1401–A1408. 10.1149/2.1011507jes. - DOI
-
- Li W.; Erickson E. M.; Manthiram A. High-Nickel Layered Oxide Cathodes for Lithium-Based Automotive Batteries. Nat. Energy 2020, 5 (1), 26–34. 10.1038/s41560-019-0513-0. - DOI
-
- Myung S. T.; Maglia F.; Park K. J.; Yoon C. S.; Lamp P.; Kim S. J.; Sun Y. K. Nickel-Rich Layered Cathode Materials for Automotive Lithium-Ion Batteries: Achievements and Perspectives. ACS Energy Lett. 2017, 2 (1), 196–223. 10.1021/acsenergylett.6b00594. - DOI
-
- Chen Y.; Song S.; Zhang X.; Liu Y. The Challenges, Solutions and Development of High Energy Ni-Rich NCM/NCA LiB Cathode Materials. J. Phys. Conf Ser. 2019, 1347 (1), 012012.10.1088/1742-6596/1347/1/012012. - DOI
-
- Schipper F.; Erickson E. M.; Erk C.; Shin J.-Y.; Chesneau F. F.; Aurbach D. Review—Recent Advances and Remaining Challenges for Lithium Ion Battery Cathodes. J. Electrochem. Soc. 2017, 164 (1), A6220–A6228. 10.1149/2.0351701jes. - DOI
LinkOut - more resources
Full Text Sources
