RAFT Dispersion Polymerization of 2-Hydroxyethyl Methacrylate in Non-polar Media
- PMID: 39741961
- PMCID: PMC11684172
- DOI: 10.1021/acs.macromol.4c02016
RAFT Dispersion Polymerization of 2-Hydroxyethyl Methacrylate in Non-polar Media
Abstract
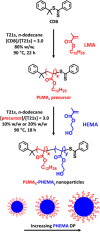
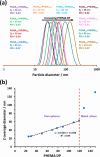
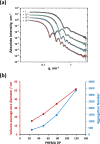
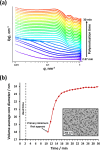
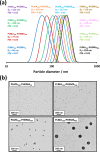
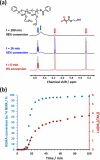
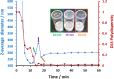
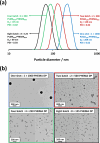
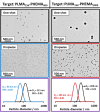
We report the reversible addition-fragmentation chain transfer (RAFT) dispersion polymerization of 2-hydroxyethyl methacrylate (HEMA) in n-dodecane using a poly(lauryl methacrylate) (PLMA) precursor at 90 °C. This formulation is an example of polymerization-induced self-assembly (PISA), which leads to the formation of a colloidal dispersion of spherical PLMA-PHEMA nanoparticles at 10-20% w/w solids. PISA syntheses involving polar monomers in non-polar media have been previously reported but this particular system offers some unexpected and interesting challenges in terms of both synthesis and characterization. First, GPC analysis requires chemical derivatization of the pendent hydroxyl groups in the PHEMA block using excess acetyl chloride to ensure that both blocks are fully soluble in chloroform. Second, DLS, TEM and 1H NMR spectroscopy studies of the periodically sampled polymerizing mixture indicate the transient formation of anomalously large, colloidally unstable aggregates at around 50% conversion, which approximately corresponds to the maximum rate of polymerization. Remarkably, such aggregates immediately break up to form well-defined nanoparticles, which remain colloidally stable at the end of the HEMA polymerization. Moreover, depending on the target degree of polymerization (DP) for the PHEMA block, TEM studies typically indicate bimodal particle size distributions for PLMA-PHEMA nanoparticles prepared using a one-shot batch protocol. This is attributed to a side-reaction between HEMA monomer and the dithiobenzoate-based RAFT agent. Fortunately, this problem can be prevented by conducting such PISA syntheses under monomer-starved conditions by continuous addition of the HEMA monomer using a syringe pump. Alternatively, unimodal spheres can also be produced via adding HEMA in multiple batches. This PISA formulation has been optimized to produce monomodal particle size distributions while targeting a PHEMA DP of up to 1000 at the maximum possible copolymer concentration. Finally, time-resolved small-angle X-ray scattering (SAXS) studies indicate the rapid formation of well-defined near-monodisperse spheres when targeting PLMA14-PHEMA50 nanoparticles.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



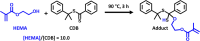

References
-
- Charleux B.; Delaittre G.; Rieger J.; D’Agosto F. Polymerization-Induced Self-Assembly: From Soluble Macromolecules to Block Copolymer Nano-Objects in One Step. Macromolecules 2012, 45, 6753–6765. 10.1021/ma300713f. - DOI
-
- Liu C.; Hong C. Y.; Pan C. Y. Polymerization Techniques in Polymerization-Induced Self-Assembly (PISA). Polym. Chem. 2020, 11, 3673–3689. 10.1039/D0PY00455C. - DOI
LinkOut - more resources
Full Text Sources
