Dehydroevodiamine Alleviates Doxorubicin-Induced Cardiomyocyte Injury by Regulating Neuregulin-1/ErbB Signaling
- PMID: 39742014
- PMCID: PMC11646148
- DOI: 10.1155/cdr/5538740
Dehydroevodiamine Alleviates Doxorubicin-Induced Cardiomyocyte Injury by Regulating Neuregulin-1/ErbB Signaling
Abstract
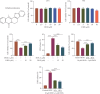
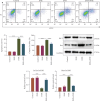
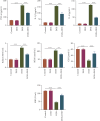
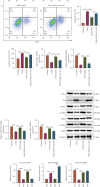
Background: Doxorubicin (DOX) is a widely used antitumor drug; however, its use is limited by the risk of serious cardiotoxicity. Dehydroevodiamine (DHE) is a quinazoline alkaloid which has antiarrhythmic effects. The aim of this study was to investigate the protective effect of DHE on doxorubicin-induced cardiotoxicity (DIC) and its potential mechanism. Materials and Methods: Rat H9c2 cardiomyocytes were exposed to DOX for 24 h to establish a DOX-induced cardiomyocyte injury model. DHE and ErbB inhibitor AG1478 were used to treat H9c2 cells to investigate their effects. Cell counting kit-8 (CCK-8) and lactate dehydrogenase (LDH) release assays were used to evaluate cell viability. Flow cytometry and caspase-3 activity assay were used to detect apoptosis. Western blot was used to detect the expression levels of apoptosis-related proteins and neuregulin-1 (NRG1)/ErbB pathway-related proteins. The levels of proinflammatory cytokines and markers of oxidative stress were also detected, respectively. Quantitative polymerase chain reaction (qPCR) was used to detect mRNA expression levels of hub genes. Results: DHE enhanced cardiomyocyte viability and decreased LDH release in a concentration- and time-dependent manner. DHE also significantly inhibited DOX-induced cardiomyocyte apoptosis, inflammation, and oxidative stress. Bioinformatics analysis showed that the protective mechanism of DHE against DIC was related to ErbB signaling pathway. DOX treatment significantly reduced NRG1, p-ErbB2, and p-ErbB4 protein expression levels in cardiomyocytes, while DHE pretreatment reversed this effect. ErbB inhibitor AG1478 reversed the protective effect of DHE on cardiomyocytes. Conclusion: DHE protects cardiomyocytes against DOX by regulating NRG1/ErbB pathway. DHE may be a potential agent for the prevention and treatment of DIC.
Keywords: NRG1/ErbB pathway; cardiotoxicity; dehydroevodiamine; doxorubicin.
Copyright © 2024 Song Jie et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Examining the protective role of ErbB2 modulation in human-induced pluripotent stem cell-derived cardiomyocytes.Toxicol Sci. 2014 Oct;141(2):547-59. doi: 10.1093/toxsci/kfu150. Epub 2014 Jul 23. Toxicol Sci. 2014. PMID: 25055963 Free PMC article.
-
Human Amnion Membrane Proteins Prevent Doxorubicin-Induced Oxidative Stress Injury and Apoptosis in Rat H9c2 Cardiomyocytes.Cardiovasc Toxicol. 2020 Aug;20(4):370-379. doi: 10.1007/s12012-020-09564-8. Cardiovasc Toxicol. 2020. PMID: 32086724
-
Acute doxorubicin cardiotoxicity is associated with miR-146a-induced inhibition of the neuregulin-ErbB pathway.Cardiovasc Res. 2010 Sep 1;87(4):656-64. doi: 10.1093/cvr/cvq148. Epub 2010 May 21. Cardiovasc Res. 2010. PMID: 20495188 Free PMC article.
-
An update of the molecular mechanisms underlying doxorubicin plus trastuzumab induced cardiotoxicity.Life Sci. 2021 Sep 1;280:119760. doi: 10.1016/j.lfs.2021.119760. Epub 2021 Jun 21. Life Sci. 2021. PMID: 34166713 Review.
-
Cytostatic drugs, neuregulin activation of erbB receptors, and angiogenesis.Curr Hypertens Rep. 2010 Dec;12(6):411-7. doi: 10.1007/s11906-010-0148-9. Curr Hypertens Rep. 2010. PMID: 20878505 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

