Channels, Transporters, and Receptors at Membrane Contact Sites
- PMID: 39742107
- PMCID: PMC11686659
- DOI: 10.1177/25152564241305593
Channels, Transporters, and Receptors at Membrane Contact Sites
Abstract
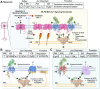
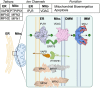
Membrane contact sites (MCSs) are specialized regions where two or more organelle membranes come into close apposition, typically separated by only 10-30 nm, while remaining distinct and unfused. These sites play crucial roles in cellular homeostasis, signaling, and metabolism. This review focuses on ion channels, transporters, and receptors localized to MCSs, with particular emphasis on those associated with the plasma membrane and endoplasmic reticulum (ER). We discuss the molecular composition and functional significance of these proteins in shaping both organelle and cellular functions, highlighting their importance in excitable cells and their influence on intracellular calcium signaling. Key MCSs examined include ER-plasma membrane, ER-mitochondria, and ER-lysosome contacts. This review addresses our current knowledge of the ion channels found within these contacts, the dynamic regulation of MCSs, their importance in various physiological processes, and their potential implications in pathological conditions.
Keywords: IP3 receptor; calcium (Ca2+); calcium-induced calcium release (CICR); endoplasmic reticulum (ER); ion channels; junctophilin (JPH); large conductance Ca2+-activated K+ channel (BKCa); membrane contact sites (MCS); neurodegeneration; niemann-Pick type C1 (NPC1); orai; ryanodine receptors (RyR); stromal interaction molecule (Stim); transient receptor potential cation channel subfamily M member 4 (TRPM4); voltage-gated calcium channel (CaV); voltage-gated potassium channel (KV).
© The Author(s) 2024.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


References
-
- Aherrahrou Z, Schlossarek S, Stoelting S, Klinger M, Geertz B, Weinberger F, Kessler T, Aherrahrou R, Moreth K, Bekeredjian R, et al. (2016). Knock-out of nexilin in mice leads to dilated cardiomyopathy and endomyocardial fibroelastosis. Basic Res Cardiol 111, 6. doi: 10.1007/s00395-015-0522-5. - PubMed
-
- Alzheimer's Association Calcium Hypothesis Workgroup and Khachaturian, Z.S (2017). Calcium Hypothesis of Alzheimer's disease and brain aging: A framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimer's Dementia: J Alzheimer's Assoc 13, 178–182 e117. doi: 10.1016/j.jalz.2016.12.006 - PubMed
-
- Apicco DJ, Shlevkov E, Nezich CL, Tran DT, Guilmette E, Nicholatos JW, Bantle CM, Chen Y, Glajch KE, Abraham NA, et al. (2021). The Parkinson’s disease-associated gene ITPKB protects against α-synuclein aggregation by regulating ER-to-mitochondria calcium release. Proc Natl Acad Sci 118, e2006476118. doi: 10.1073/pnas.2006476118 - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
