Tailoring cell-inspired biomaterials to fuel cancer therapy
- PMID: 39742146
- PMCID: PMC11683242
- DOI: 10.1016/j.mtbio.2024.101381
Tailoring cell-inspired biomaterials to fuel cancer therapy
Abstract
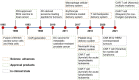
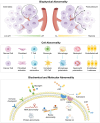
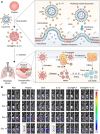
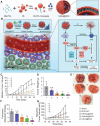
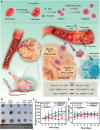
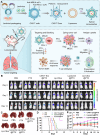
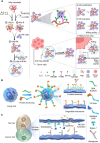
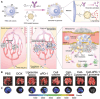
Cancer stands as a predominant cause of mortality across the globe. Traditional cancer treatments, including surgery, radiotherapy, and chemotherapy, are effective yet face challenges like normal tissue damage, complications, and drug resistance. Biomaterials, with their advantages of high efficacy, targeting, and spatiotemporal controllability, have been widely used in cancer treatment. However, the biocompatibility limitations of traditional synthetic materials have restricted their clinical translation and application. Natural cell-inspired biomaterials inherently possess the targeting abilities of cells, biocompatibility, and immune evasion capabilities. Therefore, cell-inspired biomaterials can be used alone or in combination with other drugs or treatment strategies for cancer therapy. In this review, we first introduce the timeline of key milestones in cell-inspired biomaterials for cancer therapy. Then, we describe the abnormalities in cancer including biophysics, cellular biology, and molecular biology aspects. Afterwards, we summarize the design strategies of cell-inspired antitumor biomaterials. Subsequently, we elaborate on the application of antitumor biomaterials inspired by various cell types. Finally, we explore the current challenges and prospects of cell-inspired antitumor materials. This review aims to provide new opportunities and references for the development of antitumor cell-inspired biomaterials.
Keywords: Biomaterials; Cancer therapy; Cell-inspired.
© 2024 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Wenzhi Song reports financial support was provided by International Cooperation Project of the Jilin Department of Science and Technology. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Recent developments in mussel-inspired materials for biomedical applications.Biomater Sci. 2021 Oct 12;9(20):6653-6672. doi: 10.1039/d1bm01126j. Biomater Sci. 2021. PMID: 34550125 Review.
-
Structure-optimized and microenvironment-inspired nanocomposite biomaterials in bone tissue engineering.Burns Trauma. 2024 Jun 9;12:tkae036. doi: 10.1093/burnst/tkae036. eCollection 2024. Burns Trauma. 2024. PMID: 38855573 Free PMC article. Review.
-
Piezoelectric Biomaterials Inspired by Nature for Applications in Biomedicine and Nanotechnology.Adv Mater. 2024 Aug;36(35):e2406192. doi: 10.1002/adma.202406192. Epub 2024 Jul 14. Adv Mater. 2024. PMID: 39003609 Review.
-
Biomaterials Facilitating Dendritic Cell-Mediated Cancer Immunotherapy.Adv Sci (Weinh). 2023 Jun;10(18):e2301339. doi: 10.1002/advs.202301339. Epub 2023 Apr 23. Adv Sci (Weinh). 2023. PMID: 37088780 Free PMC article. Review.
-
Diversification and enrichment of clinical biomaterials inspired by Darwinian evolution.Acta Biomater. 2016 Sep 15;42:33-45. doi: 10.1016/j.actbio.2016.06.039. Epub 2016 Jul 25. Acta Biomater. 2016. PMID: 27381524 Review.
Cited by
-
Current Research in Drug-Free Cancer Therapies.Bioengineering (Basel). 2025 Mar 26;12(4):341. doi: 10.3390/bioengineering12040341. Bioengineering (Basel). 2025. PMID: 40281701 Free PMC article. Review.
References
-
- Peer B., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nano-Enabled Med. Appl. 2020:61–91. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

