A methotrexate labelled dual metal oxide nanocomposite for long-lasting anti-cancer theranostics
- PMID: 39742148
- PMCID: PMC11683249
- DOI: 10.1016/j.mtbio.2024.101377
A methotrexate labelled dual metal oxide nanocomposite for long-lasting anti-cancer theranostics
Abstract
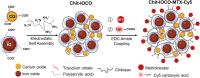
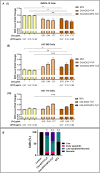
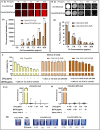
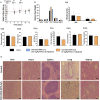
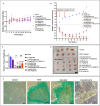
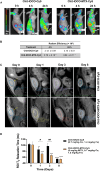
We explored the feasibility of a self-assembled chitosan nanocomposite incorporating cerium oxide/nanoceria and superparamagnetic iron oxide nanoparticles (Chit-IOCO NPs), conjugated with methotrexate (MTX) and Cy5 dye, as an integrated cancer theranostic nanosystem (Chit-IOCO-MTX-Cy5). In this system, nanoceria serves as an anti-cancer agent, while the superparamagnetic iron oxide nanoparticles function as a negative contrast agent for MR imaging. This dual metal oxide nanocomposite is conjugated with MTX which is a structural analogue of folate, serving both as a targeting mechanism for folate receptors on cancer cells and as a chemotherapeutic drug. Chit-IOCO-MTX-Cy5 exhibited exceptional negative contrast in T2 and T2∗-weighted MRI, achieving a high relaxivity of 409.5 mM⁻1 s⁻1 which is superior to clinically approved agents. The nanocomposite demonstrated both pro-oxidative and antioxidative properties, significantly increasing reactive oxygen species (ROS) production in U87MG cells (1.4-fold change), which triggered apoptosis in these cancer cells. Simultaneously, it exhibited ROS scavenging activity in non-malignant endothelial cells (0.8-fold change). Intravenous infusion of Chit-IOCO-MTX-Cy5 (5 mg/kg MTX) led to significant tumor growth inhibition, indicating a synergistic enhancement of anti-cancer effects when combining MTX and nanoceria, compared to free MTX or nanoceria without MTX conjugation. Importantly, after treatment cessation, tumours in the nanocomposite group did not re-grow, while those in the free MTX group rapidly did. In vivo MR and fluorescence imaging revealed improved uptake and retention of Chit-IOCO-MTX-Cy5 in tumours compared to nanoceria without MTX. Notably, biosafety and biochemical analyses in mice showed no significant differences between the Chit-IOCO-MTX-Cy5 treatment group and control groups.
Keywords: Cancer; Cerium oxide; Chitosan; Magnetic resonance imaging; Methotrexate; Nanoceria; Nanotheranostic; Reactive oxygen species.
© 2024 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Hang Thu Ta reports financial support was provided by 10.13039/501100000925National Health and Medical Research Council. Hang Thu Ta reports financial support was provided by 10.13039/501100001030National Heart Foundation of Australia. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Engineering chitosan nano-cocktail containing iron oxide and ceria: A two-in-one approach for treatment of inflammatory diseases and tracking of material delivery.Mater Sci Eng C Mater Biol Appl. 2021 Dec;131:112477. doi: 10.1016/j.msec.2021.112477. Epub 2021 Oct 18. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 34857262
-
Chitosan-modified manganese oxide-conjugated methotrexate nanoparticles delivering 5-aminolevulinic acid as a dual-modal T1-T2* MRI contrast agent in U87MG cell detection.MAGMA. 2024 Oct;37(5):909-924. doi: 10.1007/s10334-024-01169-4. Epub 2024 May 25. MAGMA. 2024. PMID: 38795276
-
Galactomannan armed superparamagnetic iron oxide nanoparticles as a folate receptor targeted multi-functional theranostic agent in the management of cancer.Int J Biol Macromol. 2022 Oct 31;219:740-753. doi: 10.1016/j.ijbiomac.2022.07.185. Epub 2022 Jul 28. Int J Biol Macromol. 2022. PMID: 35907463
-
Nanoceria: an innovative strategy for cancer treatment.Cell Mol Life Sci. 2023 Jan 19;80(2):46. doi: 10.1007/s00018-023-04694-y. Cell Mol Life Sci. 2023. PMID: 36656411 Free PMC article. Review.
-
Redox Active Cerium Oxide Nanoparticles: Current Status and Burning Issues.Small. 2021 Dec;17(51):e2102342. doi: 10.1002/smll.202102342. Epub 2021 Aug 6. Small. 2021. PMID: 34363314 Review.
Cited by
-
Nanomaterials in cancer starvation therapy: pioneering advances, therapeutic potential, and clinical challenges.Cancer Metastasis Rev. 2025 May 10;44(2):51. doi: 10.1007/s10555-025-10267-1. Cancer Metastasis Rev. 2025. PMID: 40347350 Free PMC article. Review.
-
Study of caspase-6 activity in aggressive HCT116 cells using methotrexate-encapsulated lactoferrin-conjugated solid lipid nanoparticles via in silico and in vitro approaches.Sci Rep. 2025 Jul 1;15(1):20775. doi: 10.1038/s41598-025-08089-w. Sci Rep. 2025. PMID: 40594879 Free PMC article.
References
-
- Funkhouser J. Reinventing pharma: the theranostic revolution. Curr. Drug Discov. 2002:17–19.
-
- Moonshi S.S., Vazquez-Prada K.X., Adelnia H., Westra van Holthe N.J., Wu Y., Tang J., Bulmer A.C., Ta H.T. Polysuccinimide-based nanoparticle: a nanocarrier with drug release delay and zero burst release properties for effective theranostics of cancer. Appl. Mater. Today. 2024;37
LinkOut - more resources
Full Text Sources

