Ocular adverse events associated with antibody-drug conjugates: a comprehensive pharmacovigilance analysis
- PMID: 39742259
- PMCID: PMC11685049
- DOI: 10.3389/fimmu.2024.1495137
Ocular adverse events associated with antibody-drug conjugates: a comprehensive pharmacovigilance analysis
Abstract
Introduction: Antibody-drug conjugates (ADCs) are increasingly utilized in patients with solid tumors and hematologic malignancies. However, the adverse ocular toxicity induced by ADCs has not been comprehensively evaluated in real-world clinical settings.
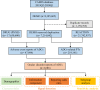
Methods: Data from April 2019 to March 2024 based on the FDA Adverse Event Reporting System (FAERS) were extracted and analyzed. Disproportionality analysis was used to evaluate the association between ADCs and ocular adverse events (AEs). The median time to onset (TTO) of various ADCs was compared.
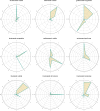
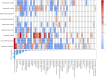
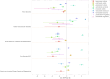
Results: A comprehensive analysis identified 2,686 ocular AEs associated with ADCs. Among these, Tisotumab vedotin had the most positive signals at the preferred terms (PTs) level, followed by trastuzumab emtansine and enfortumab vedotin. In contrast, gemtuzumab ozogamicin demonstrated minimal ocular toxicity signals. Cluster analysis revealed that ADC-related ocular toxicities predominantly manifested as corneal disorders or ocular neuromuscular disorders. The median onset of ocular toxicity varied considerably, with enfortumab vedotin showing the earliest median onset at 12.5 days.
Conclusions: Our study demonstrates the association between ADCs and ocular AEs based on real-world data, providing valuable guidance for clinicians when prescribing ADCs. And we found some important safety signals that have not been mentioned in the label or previous studies.
Keywords: FAERS; antibody-drug conjugates; ocular adverse events; pharmacovigilance; real-world study.
Copyright © 2024 Chen, He, Huang, Hu and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

