Longevity of antibody responses is associated with distinct antigen-specific B cell subsets early after infection
- PMID: 39742271
- PMCID: PMC11686410
- DOI: 10.3389/fimmu.2024.1505719
Longevity of antibody responses is associated with distinct antigen-specific B cell subsets early after infection
Abstract
Introduction: Upon infection, T cell-driven B cell responses in GC reactions induce memory B cells and antibody-secreting cells that secrete protective antibodies. How formation of specifically long-lived plasma cells is regulated via the interplay between specific B and CD4+ T cells is not well understood. Generally, antibody levels decline over time after clearance of the primary infection.
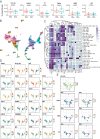
Method: In this study, convalescent individuals with stable RBD antibody levels (n=14, "sustainers") were compared with donors (n=13) with the greatest antibody decline from a cohort of 132. To investigate the role of the cellular immune compartment in the maintenance of antibody levels, SARS-CoV-2-specific responses at 4 to 6 weeks post-mild COVID-19 infection were characterized using deep immune profiling.
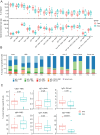
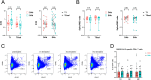
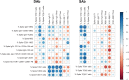
Results: Both groups had similar frequencies of total SARS-CoV-2-specific B and CD4+ T cells. Sustainers had fewer Spike-specific IgG+ memory B cells early after infection and increased neutralizing capacity of RBD antibodies over time, unlike the declining group. However, declining IgG titers correlated with lower frequency of Spike-specific CD4+ T cells.
Conclusion: These data suggest that "sustainers" have unique dynamics of GC reactions, yield different outputs of terminally differentiating cells, and improve the quality of protective antibodies over time. This study helps identify factors controlling formation of long-lived PC and sustained antibody responses.
Keywords: CD4+ T cells; SARS-CoV-2; declining/sustained antibody titers; deep-phenotyping; neutralization.
Copyright © 2024 Kuijper, Kreher, Elias, Claireaux, Kerster, Bos, Duurland, Konijn, Paul, de Jong, de Jongh, Steenhuis, Garcia-Vallejo, van Gils, Kuijpers, Eftimov, Rispens, van der Schoot, van Ham and ten Brinke.
Conflict of interest statement
Author AP was employed by Cytek Biosciences, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Piccoli L, Park YJ, Tortorici MA, Czudnochowski N, Walls AC, Beltramello M, et al. . Mapping neutralizing and immunodominant sites on the SARS-coV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell. (2020) 183:1024–1042.e21. doi: 10.1016/j.cell.2020.09.037 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

