In-depth analysis of serum antibodies against Epstein-Barr virus lifecycle proteins, and EBNA1, ANO2, GlialCAM and CRYAB peptides in patients with multiple sclerosis
- PMID: 39742283
- PMCID: PMC11685087
- DOI: 10.3389/fimmu.2024.1487523
In-depth analysis of serum antibodies against Epstein-Barr virus lifecycle proteins, and EBNA1, ANO2, GlialCAM and CRYAB peptides in patients with multiple sclerosis
Abstract
Background: A strong association between multiple sclerosis (MS) and Epstein-Barr virus (EBV) has been established but the exact role of EBV in MS remains controversial. Recently, molecular mimicry between EBNA1 and specific GlialCAM, CRYAB and ANO2 peptides has been suggested as a possible pathophysiological mechanism. The aim of this study was to analyse anti-EBV antibodies in MS patients against (I) EBV lifecycle proteins, (II) putative cross-reactive peptides, and (III) during treatment.
Methods: In this retrospective cross-sectional study, 258 serum samples were included consisting of EBV-negative (n = 25) and EBV-positive (n = 36) controls, 192 MS samples including untreated relapsing-remitting MS (RRMS) with and without relapses, secondary progressive MS (SPMS) and primary progressive MS (PPMS) patients, and 106 patients on 8 different treatment regimens. IgG and IgM antibody titers against EBV docking/fusion proteins (gp350, gh/gp42, gh/gL/gp42), immediate early antigen (BZLF1), early antigens (EA p85, EA P138, EA P54), capsid antigens (VCA P18, VCA P23, VCA gp125) and late antigens (EBNA1) were measured. Specific EBNA1 and GlialCAM, CRYAB and ANO2 peptides were synthesized and also incorporated in our custom magnetic bead based multiplex assay.
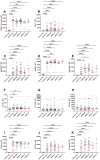
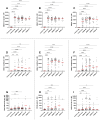
Results: We observed significantly elevated IgG antibody titers in EBV-positive controls, RRMS with and without relapse, SPMS and PPMS patients for all lifecycle antigens except for several early antigens when compared to EBV-negative controls. Significantly higher IgG antibody titers were observed in RRMS patients for fusion proteins and EBNA1 peptides when compared to EBV-positive controls. An MS specific response was observed for ANO2 but not for GlialCAM or CRYAB. No significant treatment effects or a specific IgM response were detectable.
Conclusion: The MS-specific, differential antibody response to EBV antigens confirms an altered immunological response to EBV in MS patients. EBV reactivation does not appear to play an important role in MS pathogenesis and no differential antibody signatures were observed between MS disease phases. The MS-specific anti-ANO2 antibody response suggests a potential role for EBNA1 as an antigenic driver, although the exact role of anti-ANO2 antibodies needs to be determined. The precise pathophysiological role of EBV in MS remains uncertain and requires further investigation.
Keywords: EBV nuclear antigen type 1 (EBNA1); Epstein-Barr virus (EBV); alpha B crystallin (CRYAB); anoctamin 2 (ANO2); glial cell adhesion molecule (GlialCAM); multiple sclerosis (MS).
Copyright © 2024 Vasilenko, Tieck, Michel, Schembecker, Schwarz, Guenther, Ruschil, Poli, Ziemann, Giede-Jeppe, Gabernet, Dulovic and Kowarik.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Besson C, Amiel C, Le-Pendeven C, Brice P, Fermé C, Carde P, et al. . Positive correlation between epstein-barr virus viral load and anti-viral capsid immunoglobulin G titers determined for hodgkin’s lymphoma patients and their relatives. J Clin Microbiol. (2006) 44:47–50. doi: 10.1128/JCM.44.1.47-50.2006 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

