Novel inhibitory effect of Omega-3 fatty acids regulating pancreatic cancer progression
- PMID: 39742417
- PMCID: PMC12097994
- DOI: 10.1093/carcin/bgae081
Novel inhibitory effect of Omega-3 fatty acids regulating pancreatic cancer progression
Abstract
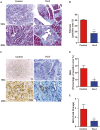
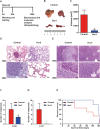
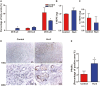
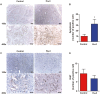
Pancreatic cancer is a devastating malignancy in great need of new and more effective treatment approaches. In recent years, studies have indicated that nutritional interventions, particularly nutraceuticals, may provide novel avenues to modulate cancer progression. Here, our study characterizes the impact of ω-3 polyunsaturated fatty acids, eicosapentaenoic acid, and docosahexaenoic acid, as a nutraceutical intervention in pancreatic cancer using a genetically engineered mouse model driven by KrasG12D and Trp53R172H. This model closely resembles human pancreatic carcinogenesis, offering a disease relevant platform for translational research. Our findings showed that ω-3 polyunsaturated fatty acids intervention (using a diet supplemented with 6% cod liver oil) significantly reduced tumor volume as well as lung and liver metastasis and a trend toward improved survival rate compared with control treated mice. This antitumoral effect was accompanied by distinct changes in tumor membrane fatty acid profile and eicosanoids release. Furthermore, the eicosapentaenoic acid and docosahexaenoic acid intervention also reduced malignant histological parameters and induced apoptosis without affecting cell proliferation. Of note is the significant reduction in tumor fibrosis that was associated with decreased levels of Sonic Hedgehog, a major ligand controlling this cellular compartment in pancreatic cancer. All together our results demonstrate the impact of eicosapentaenoic acid and docosahexaenoic acid as antitumor regulators in pancreatic cancer, suggesting potential for ω-3 polyunsaturated fatty acids as a possible antitumoral dietary intervention. This research opens new avenues for integrating nutraceutical strategies in pancreatic cancer management.
Keywords: Sonic Hedgehog; nutraceutical intervention; pancreatic cancer; ω-3 polyunsaturated fatty acids.
© The Author(s) 2025. Published by Oxford University Press. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
None declared.
Figures


References
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12–49. https://doi.org/10.3322/caac.21820 - DOI - PubMed
-
- Suda A, Umaru BA, Yamamoto Y et al. Polyunsaturated fatty acids-induced ferroptosis suppresses pancreatic cancer growth. Sci Rep 2024;14:4409. https://doi.org/10.1038/s41598-024-55050-4 - DOI - PMC - PubMed
-
- Wirkus J, Ead AS, Mackenzie GG. Impact of dietary fat composition and quantity in pancreatic carcinogenesis: recent advances and controversies. Nutr Res 2021;88:1–18. https://doi.org/10.1016/j.nutres.2020.12.018 - DOI - PubMed
-
- Hingorani SR, Wang L, Multani AS et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005;7:469–83. https://doi.org/10.1016/j.ccr.2005.04.023 - DOI - PubMed
-
- Dawson DW, Hertzer K, Moro A et al. High-fat, high-calorie diet promotes early pancreatic neoplasia in the conditional KrasG12D mouse model. Cancer Prev Res (Phila) 2013;6:1064–73. https://doi.org/10.1158/1940-6207.CAPR-13-0065 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R01 CA265050/CA/NCI NIH HHS/United States
- 2018-2020: 411/18, 455/18 472/18/Secretaría de Ciencia y Tecnología de la Universidad Nacional de Córdoba
- #22920180100043CO/Consejo Nacional de Investigaciones Científicas y Técnicas - Argentina
- FONCYT-PICT-2021-GRFTI-00543/Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación
LinkOut - more resources
Full Text Sources
Medical

